Chromatography
What colors make up black marker ink?

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Simply put it back as quickly as possible. Don’t consider this a failure! You can always repeat the experiment with a new piece of filter paper and reuse the wet cotton cylinder.
Try adding some more NaHSO4 solution to the cotton cylinder (10-20 drops).
Yes, but the results will differ. Water won’t separate the colors quite as well - you will achieve the desired result, but not as distinctly as with a strong acid like sodium hydrogen sulfate NaHSO4.
This experiment works for any marker containing a combination of various dyes. Try them out! Brown and blue markers will normally yield an interesting result. Check out purple and orange as well! Just keep in mind that the result will depend heavily on the marker manufacturer.
You can try citric acid, but it usually doesn’t work quite as well. A solution of hydrochloric acid will work much better, but be extremely careful and follow all safety precautions while working with strong acids!
And, of course, you can try using water! It works more slowly and separates the dyes less effectively, but on the other hand, you can repeat using water as many times as you like.
Step-by-step instructions
Black markers are filled with black dye. Or are they?
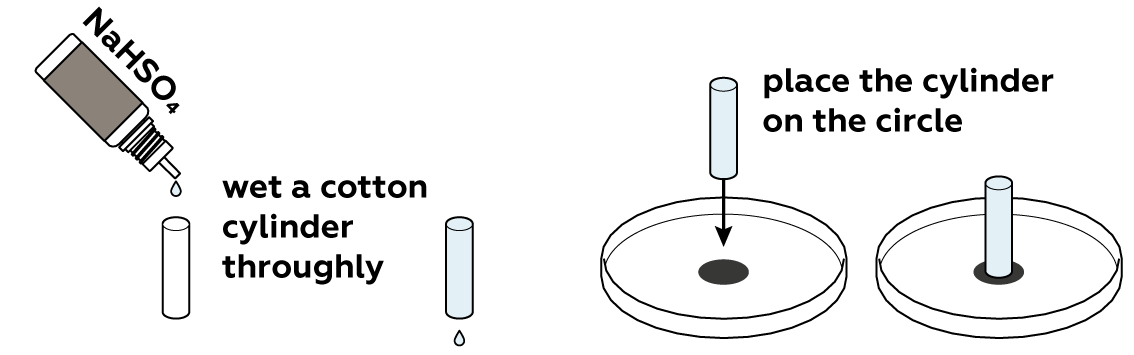
Since the dye in the marker is water-soluble, we will perform our test using an aqueous solution. We will use an acidic solution of sodium hydrogen sulfate, but other aqueous solutions or even clear water would also work to some extent.
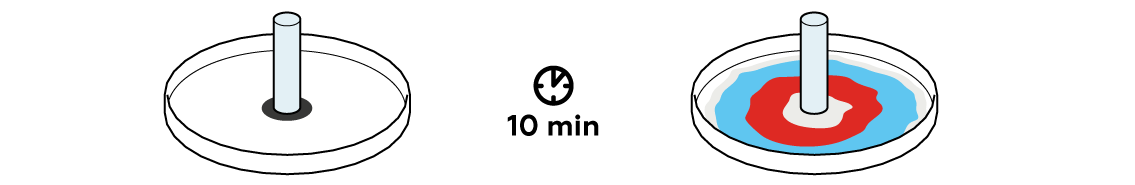
Our solvent dissolves the ink that the circle was drawn with and spreads through the filter paper, taking the ink with it. Let’s see what’s going to happen in this process.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Why do the colors separate?
The ink in the black marker consists of several pigments, which are dissolved in a solvent containing some water and alcohol. At the end of the experiment, we see these pigments separated on the filter paper.
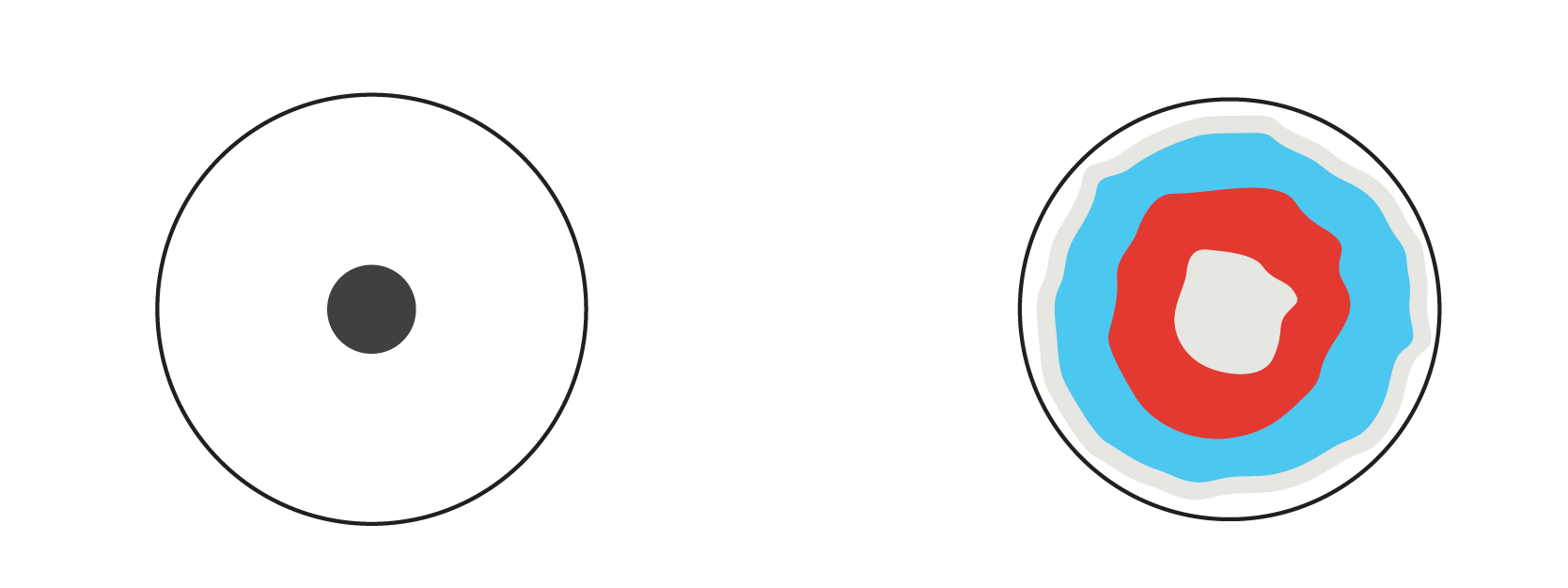
The black circle is moistened with sodium hydrogen sulfate NaHSO4 and gradually spreads along the paper. The pigments making up the black ink move with the solution as well, but since these pigments are different in nature, they move at varying speeds. This results in multicolored rings!
How can a few colored dyes make black when mixed?
We should start with two key concepts: what is light and what is color. Light is actually an electromagnetic emanation or EMI. This is a rather complex topic, but mostly we need to understand that light is something that objects (and, for example, our eyes) can interact with. An object’s color is the result of such interactions.
We usually consider light sources to be white light (sunlight, or light from a lamp). In reality, any more-or-less white light is a mixture of the whole spectrum of colored light.
There are two interactions between light and objects we need to consider: the absorption and reflection of light. When an object reflects all the white light hitting it, our eyes perceive the object to be white. When an object absorbs all the light, we don’t see any reflected light from this object and it seems black.

Colored things like dyes, in turn, absorb some colors more than others, and as a result, we see some of the light reflected back. But this kind of object doesn’t look white - it can be red, blue, yellow, or a multitude of other colors!
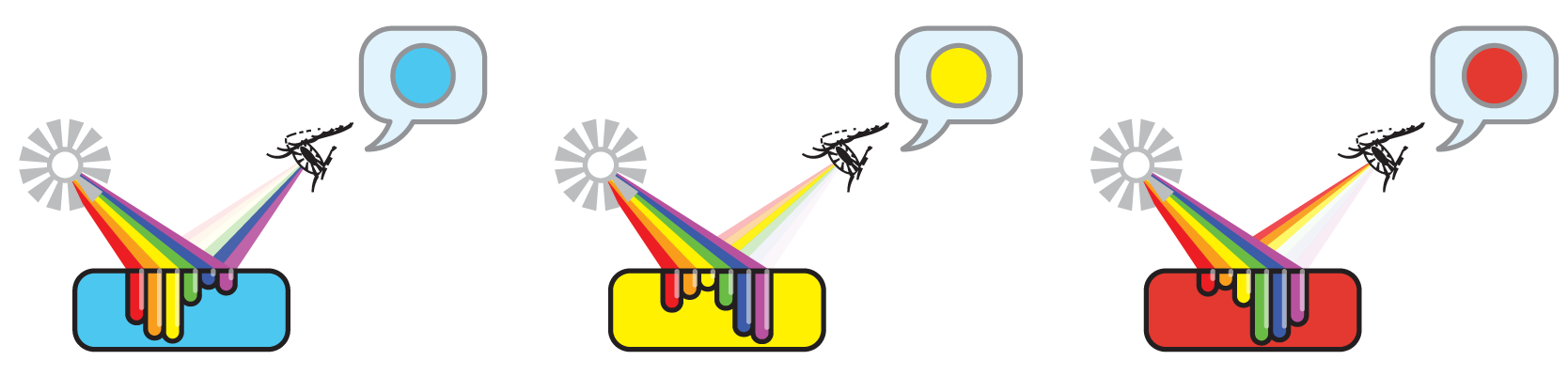
And thus if we mix some differently-colored dyes, they can, together, absorb all the light hitting them, leaving our eyes without any reflected light from this mixture. In other words, a mixture of vividly-colored dyes can be black, just as in the experiment!
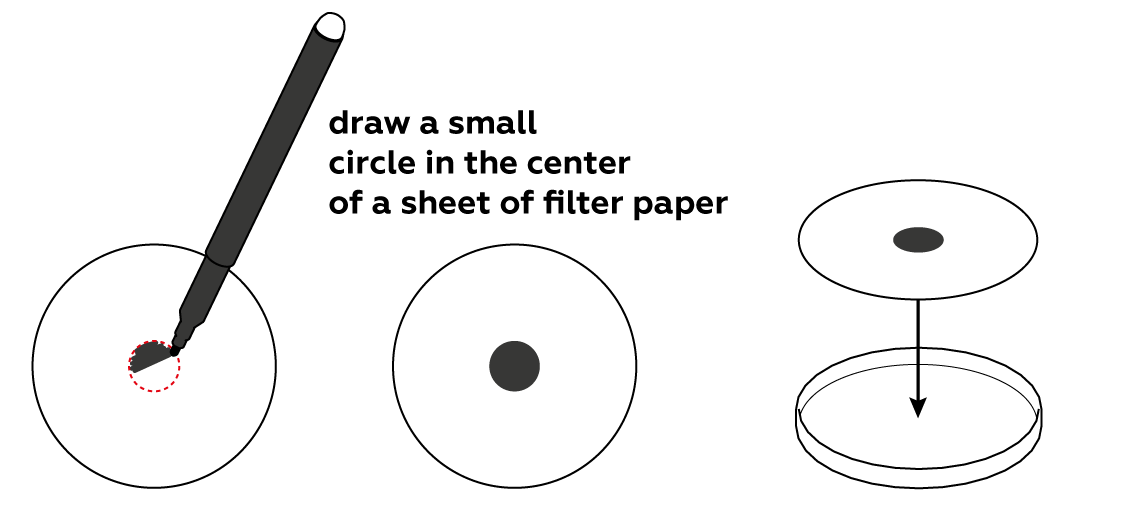
Is it important to place the black circle in the middle of the paper sheet?
Yes; the dissolved inks will spread along the paper evenly only if we start from the middle. Otherwise, the experiment will work, but differently, and it will be harder to evaluate the result. But try it out!
What do we need NaHSO4 solution for?
Sodium hydrogen sulfate NaHSO4 is a substance that can make a solution very acidic. In other words, the pH of a solution containing NaHSO4 will be very low. As it turns out, the pigments in black ink separate more easily on the filter paper in acidic conditions.
The method of separating substances according to their interactions with the solution they move with and the surface they spread through is one of the various kinds of chromatography.
What is this method?
Chromatography is used to separate and analyze substances in complex mixtures or in a solution. The substances we analyze interact with two phases - stationary and mobile. According to their differences in structure, substances can interact with both phases in different ways. Thus, they separate and we can analyze them one by one, rather than in combination.
In our experiment, the filter paper acts as the stationary phase, while the sodium hydrogen sulfate NaHSO4 solution is the mobile phase. The different pigments interact differently with the sodium hydrogen sulfate solution and with the paper’s surface. Consequently, they move at different speeds and we get our result, separating the black ink into red and blue substances.
That’s interesting!
Chromatography is a technique used to separate molecules based on differences in their speeds when they move in specific surroundings. An individual molecule’s speed depends on its size, polarity, and interaction forces with a solvent and a stationary phase.
How does it work? In principle, chromatography is reminiscent of an ocean wave that washes pebbles and seashells ashore. Tiny debris drifts further onto the shore, while larger objects get stuck closer to the waterline. In chromatography, molecules move along with the solvent atop a stationary phase, just as pebbles ride a wave onto the shore. Here, our solvent acts as the ocean wave that separates molecules depending on their size by carrying them greater or lesser distances. The sheet of paper represents our imaginary shore, and a solution of sodium bisulfate - the ocean wave.
This method was developed by Mikhail Tsvet as a means to sort molecules in a solution based on their molecular mass. Later, this technique was expanded to separate molecules depending on their charge, affinity to a solvent, or behavior in an electric field.
Chromatography isn’t limited to laboratories. This same principle works in pregnancy tests and many other dipstick tests that detect different substances in liquids. Various medical tests are also based on chromatography.
There are over one hundred distinct chromatography methods. Modern instruments allow us to detect about 600 compounds in a solution! For instance, this technique is extensively used to analyze crude petroleum.
The main type of chromatography we use today is a gas-liquid chromatography. Here, liquid acts as a stationary phase, and gas as a mobile phase. This method allows us to separate complex mixtures containing hundreds of components!