Nitrogen atom
An interactive lab where students assemble their own nitrogen atom given only the number of electrons.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript






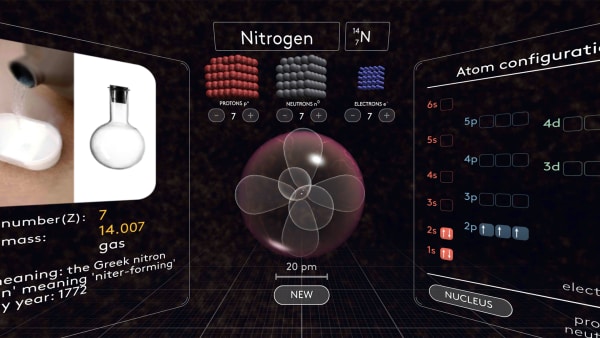
Teacher's notes
Keywords
nitrogen, atom, nucleus, proton, neutron, isotope, electron, electron orbital, electron configuration diagram, s-orbital, p-orbital
Students will
- Build a nitrogen atom knowing only the number of electrons
- Review electroneutrality and calculate how many protons a nitrogen atom has
- Make an educated guess about how many neutrons a nitrogen atom might have
- Review electron orbital names, shapes, and order of appearance
- Review electron configuration diagrams
The lab serves primarily as a skill builder for electron configuration. It can be used in a variety of topics throughout the curriculum: periodicity and properties, covalent bonding (triple bond in nitrogen molecule), introduction and basic concepts in organic chemistry (amines and their structure).
Topics to discuss
- Nitrogen in the Earth's atmosphere