Detective Iodine
Reveal fingerprints using iodine!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray and in a well-ventilated area.
- Avoid inhaling iodine vapors from the cup.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
If your fingers are too dry, your fingerprints will not appear. Your fingerprints appear when grease from your skin interacts with iodine vapor. Wipe your forehead one more time (this will make your fingers greasier) and try the experiment again.
Getting a good print isn’t easy, even for a born detective! If your fingers are too greasy, the fingerprints will appear as amorphous and ambiguous stains. Wash your hands and dry them thoroughly. Rub your forehead slightly with the fingers you want to take fingerprints from. Then rub your fingertips together. Press your fingers firmly on the paper for 2-3 seconds, but try not to move them along the paper, otherwise the image will smudge. Try to develop these new fingerprints with the iodine vapor from the disposable cup.
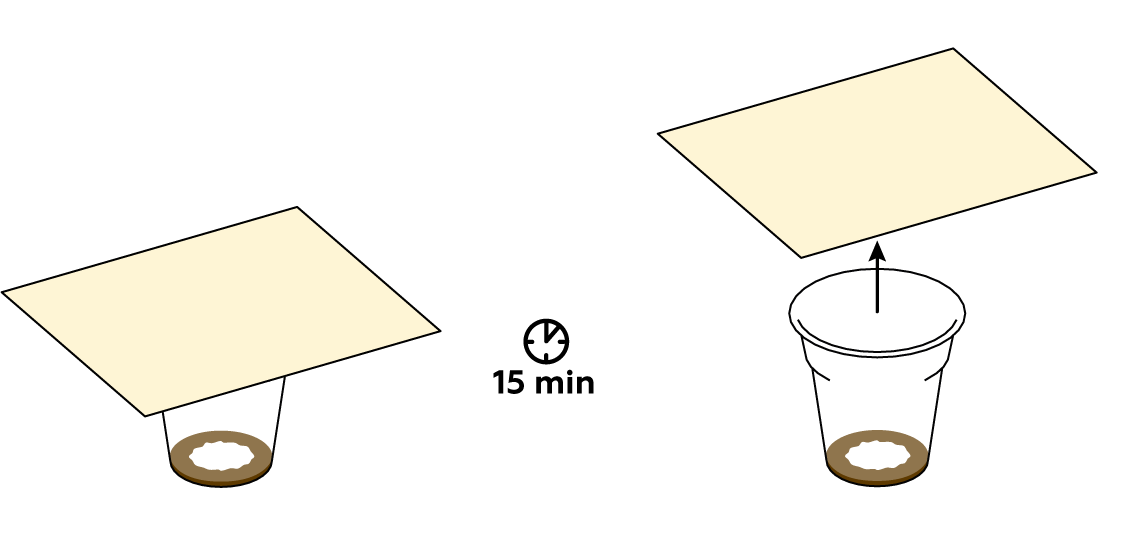
First of all, make sure that the mixture in the plastic cup is brown. If not, try adding some more СuSO4 crystals and KI solution. Then put the paper on top of the cup again, fingerprint side down. The colder the room, the slower the iodine will evaporate: if you’re working in the cold, try giving the reaction a bit more than 15 minutes. If nothing happens, try the experiment again from the beginning.
Yes, of course, you can try using ordinary white paper for this experiment.
Unfortunately, you won't be able to easily keep the prints looking good for a long time. Iodine is a volatile substance and it will evaporate quickly.
Step-by-step instructions
Potassium iodide KI contains iodide ions I-, which we can turn into iodine molecules I2 using CuSO4.
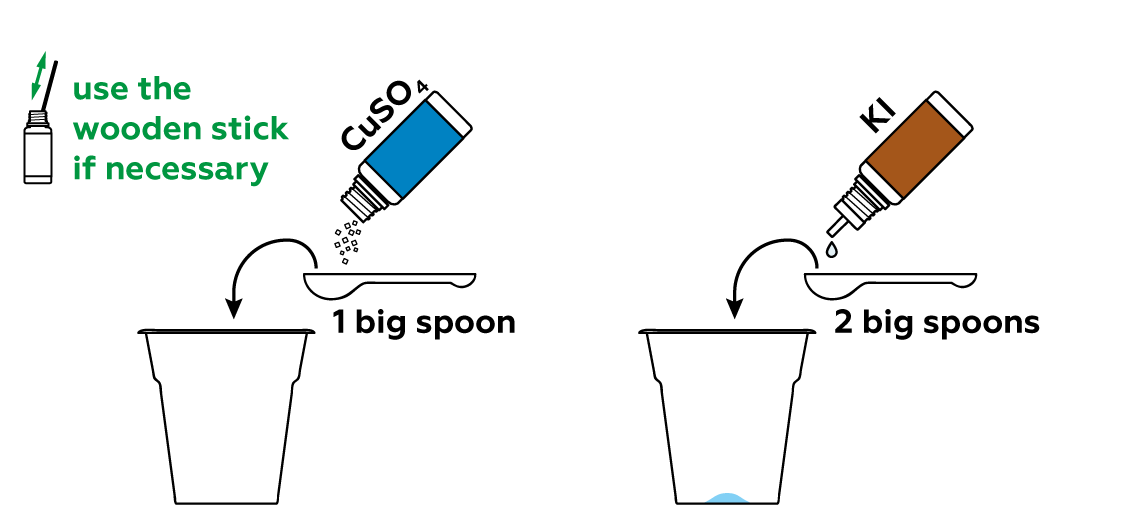
Unlike criminals, we want to be sure to leave some fingerprints.
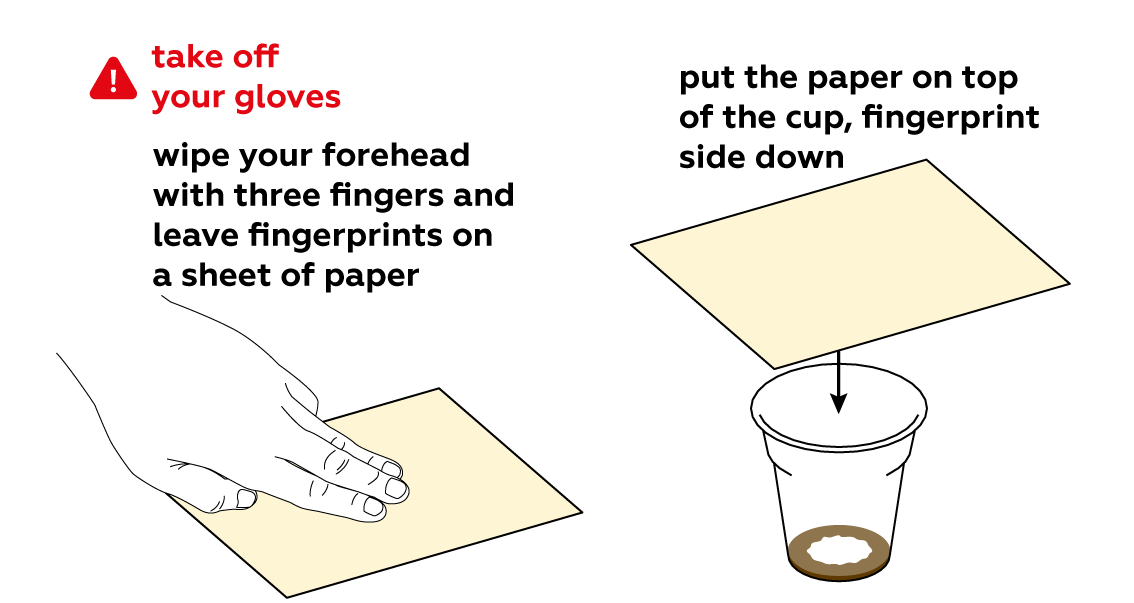
Iodine is quite volatile. This means that its molecules leave the solution easily and disperse in the form of a purple gas.
Iodine sticks to fats really well, and our fingerprints consist mostly of fats.

Expected result
Iodine vapors are absorbed by fats contained in the fingerprints left on the paper. Iodine reveals the fingerprints by coloring them characteristic brown.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description
Potassium iodide KI contains iodide ions I-
, which are basically iodine atoms
with an extra electron
, and CuSO4 contains a Cu2+ ion
, a copper atom
with two electrons missing. Cu2+ can take an electron from I-
, turning it into I0
. The latter likes to bond in pairs, forming iodine molecules I2
. When these iodine molecules form, our solution turns brown.
Iodine is rather volatile and can easily escape a solution. But there are plenty of things all around that iodine molecules can stick to. For example, iodine dissolves very well in fats, and fats are what fingerprints are mostly made of. Even the small amount of fat from our fingers can host enough iodine molecules to make otherwise colorless fingerprints dark brown and clearly visible.
Even though there are plenty of other methods for revealing invisible fingerprints, this one is particularly helpful when dealing with rough and porous surfaces like paper. Forensics experts use it to help catch greasy-fingered criminals even today.
How does iodine form?
Potassium iodide KI interacts with copper sulfate CuSO4:
2CuSO4 + 4KI → I2 + 2CuI + 2K2SO4
In the solution, both of these initial substances split into ions:
KI → K+ + I–
CuSO4 → Cu2+ + SO42–
The copper ions Cu2+ oxidize the iodide ions I– by taking an electron:
Cu2+ + I– → Cu+ + I0
As a result, molecular iodine (I2) forms:
I0 +I0 → I2
Why does the fingerprint discolor so quickly?
The color of the fingerprint is determined by the presence of the molecular iodine that binds to the oils in the secretions from the skin. However, these bonds are rather weak — the iodine eventually sublimes. This causes the fingerprint to discolor over the course of about 15–30 minutes.
The process we encounter in the experiment is reversible. In the beginning, we see that our plastic cup contains a lot of iodine, while the fingerprint contains none. So, during a process called diffusion, in which the particles of any particular substance tend to spread out to more or less evenly fill a space, iodine molecules enter the fingerprint. Later, when we invariably leave our iodine-infused fingerprint exposed to air (which contains either no or very few iodine molecules), the iodine molecules leave the fingerprint and enter the air, trying to distribute themselves evenly between the air and the fingerprint. But since there is a seemingly endless amount of air compared to the volume of the fingerprint, most or all of the iodine molecules abandon the fingerprint and venture out into the open air.
Your fingerprint will eventually fade as the iodine disappears. The same process can be observed with iodine on the skin. It is often used as a disinfectant for scrapes and cuts, and many people think that the iodine stain on the skin disappears as the iodine is absorbed by the body. Indeed, a small fraction of the iodine molecules are absorbed, but the majority simply sublime directly from the surface of the skin into the atmosphere.
Is there any way to preserve the print’s color?
The fingerprint’s color can be preserved by depositing a thin layer of highly reactive carbonyl iron (Fe) powder directly onto the fingerprint. This prevents the iodine from escaping from the fingerprint and returning to the atmosphere; instead, the iodine reacts with the carbonyl iron (Fe).
Fe + I2 → FeI2
This reaction yields iron iodide (FeI2), which is a stable reddish-brown compound. Due to its propensity for such reactions, iodine cannot be used to reveal fingerprints on metal surfaces, as it would react with the metal and obliterate the fingerprint rather than reveal it.
Iodine fuming has been used to develop fingerprints since the beginning of the 20th century. Though the same basic principles apply, the equipment used to conduct the process has changed somewhat over the years. In the following video, you can observe a variety of tools used by professionals today. Although the equipment does not resemble a traditional camera, the chemistry behind both procedures is the same.
Follow up
Fingerprints on fabric
Try to reveal fingerprints on a piece of white cloth instead of paper!
That’s interesting!
What is forensic science?
Forensic science encompasses a number of different methods that can help investigate crimes! The more clues a detective can find, the more successful an investigation will be. It is crucial to collect, preserve, and analyze everything that can reveal the details of a crime. Any objects of interest are studied in a laboratory. And chemistry plays a critical role in this work!
As each person’s fingerprints are unique, finding and developing fingerprints can aid an investigation quite a lot! And using iodine, as you did in the experiment, is a method that real forensic scientists apply in their work!
But what if forensic scientists need to find other clues aside from fingerprints? UV light can help, too. Applying this light to a crime scene can help reveal traces of blood, saliva or other materials of organic origin invisible to the naked eye!