Electron configuration
An electron configuration diagram is a useful tool to represent electrons in atoms. In this lesson, we will introduce this diagram and see how it works when electrons are added to the nucleus one by one. Students will also learn Hund's rule.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

You have learned that electrons occupy specific orbitals.
Today we will use a special diagram that will help us to understand the structure of electron orbitals.

Let's take our sulfur atom and check its electron structure again, this time using this diagram.
Ready to dive?
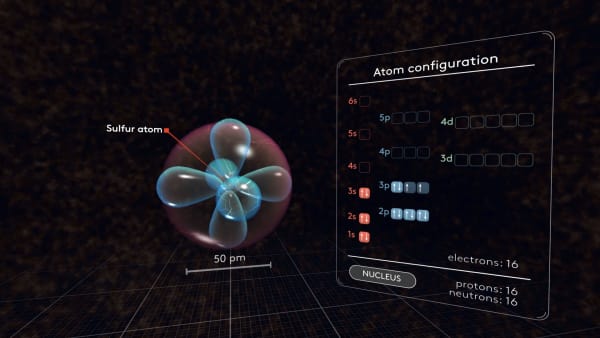
Here is our sulfur atom with all its electrons.
And here is the diagram showing all electron orbitals as small boxes.

Once again let's peel off all the electrons and add them back one by one, this time using our diagram.
Let's add our first electron — it will go to the first orbital that is called 1s.

Now look at the diagram — we add an arrow to the 1s box to show that we have one electron on this orbital.
Now let's add the second electron — as you may remember it will go to the same orbital. Now look at the diagram, we have added the second arrow to the first box to show that there are two electrons on this orbital.

Do you remember, we learned that only two electrons can be on one orbital but did not explain why? Now it is time to explain this rule.
Each electron has a property called spin that can have only one of two possible values. On our diagram, we show spin as either an up or down arrow.
The fundamental laws of physics say that two electrons cannot exist in the same state. So, if they are in the same orbital they must have different spins. But there are only two possible spin values.

Now let's add more electrons and see how they are shown in the diagram.
The next two electrons can fit in the second orbital, called 2s.

The next three electrons will occupy three different p orbitals, one electron per orbital because electrons repel each other.
Look at the diagram; we have three electrons in three boxes corresponding to our three different p orbitals.

Let's continue adding electrons and see the changes in the diagram.
When an electron is added to an orbital, we add an arrow to the corresponding box on the diagram.
Now our sulfur atom is complete. The diagram represents its electron structure.

You can play with the diagram by clicking on different boxes to see the corresponding electron orbitals.

Let's go back to our laboratory. Try to recall the name of the first orbital.
The first orbital is called 1s.
Teacher's notes
Keywords
atoms, electrons, electron orbitals, electron configuration, atom configuration, electron configuration diagram
Students will
- Recall that electrons in atoms occupy electron orbitals
- Learn that there is a convenient way to write down an electron configuration by means of an electron configuration diagram
- Examine a sulfur atom and diagram how electrons appear to occupy orbitals
Topics to discuss
- Rules for electrons: Pauli Exclusion Principle, Aufbau Principle, & Hund's Rule
- Electron configuration diagrams and the periodic table
Questions
- How to complete an electron configuration diagram for different atoms?
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.