Color-changing milk
Experiment with milk, dyes, and liquid soap!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
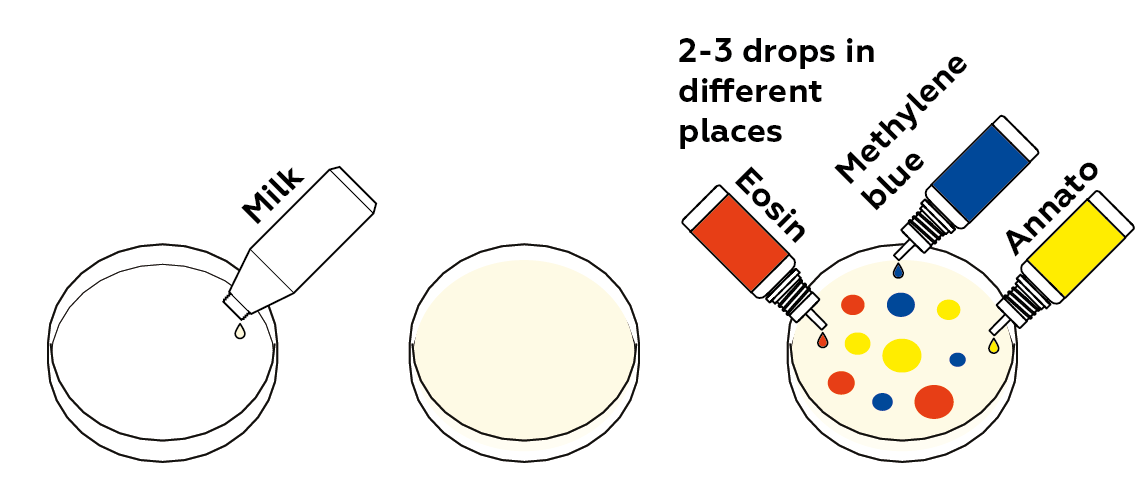
Add just as much milk as needed to cover the bottom of the Petri dish. Be careful not to add too much – you’ll mix the contents of the Petri dish as part of the next step.
Great idea! You can touch different places in the Petri dish using the soapy cotton swab. You can even try touching the cotton swab to the concentrated dots of dye themselves. You can also put the absorbent in the final mixture and see what happens!
This kit contains enough liquid soap to repeat this experiment multiple times. We don't recommend that you use the liquid soap you have at home, as the results probably won’t be quite as spectacular. Of course, you can still try using your own liquid soap – just remember to wash the Petri dish thoroughly after each attempt.
Yes, feel free to use the food coloring you have at home! If a coloring is very viscous, use water to dilute it to a suitable consistency; if a coloring is powdered, prepare an aqueous solution.
Since some food colorings dissolve poorly in water, their commercial forms may contain surface active agents, sometimes even soap, to improve their solubility. Unfortunately, such colorings won’t work for this experiment: if you add them to your milk, the other food colorings will “scatter” immediately. Test various colorings to figure out which ones are suitable for the experiment.
Yes, in principle. However, the result will look slightly different because milk and water have different physical and chemical properties.
Actually, the main reason we recommend using milk is to make the experiment more visually appealing. Compared to colorless water, vibrant colorings on a white background look much more spectacular.
The annatto in the kit is a chemical reagent – DO NOT consume it in any capacity.
Step-by-step instructions
Apply several drops of dye to the milk’s surface.
The liquid soap “runs” along the milk’s surface very fast, dragging the dyes along with it.
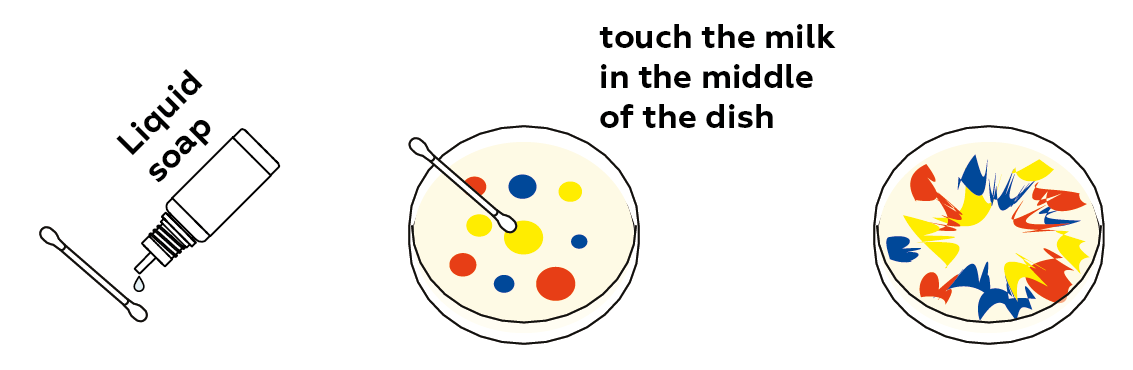
Try adding a drop of liquid soap.
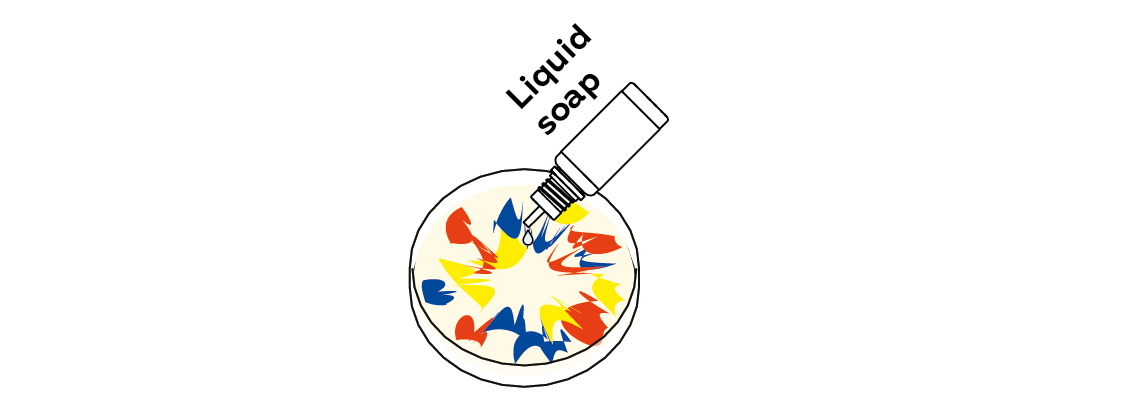
Expected result
The soap spreads to form a thin film over the surface of the milk, creating stunning patterns in the dye.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
What is the role of soap in this experiment?
As soon as the soap touches the milk, it starts to spread, and soon a very thin film of soap develops on the milk’s surface. The soap causes the colors to spread along with it, which produces the beautiful effect you see.
Soap is a tricky substance. Its molecules consist of two parts: a hydrophilic (water-loving) "head" that is strongly attracted to water, and a hydrophobic (water-fearing) "tail" that doesn't like water at all.
When soap is added to water (or milk), its molecules arrange themselves so that their heads are in the water while their tails are not. We can observe this process as the formation of a film on the surface of the liquid.
Molecules of soap and other similar substances (those that also have hydrophobic “tails” and hydrophilic “heads”) lower the surface tension of different liquids and thus are called surface-active agents (SAA). These substances help us wash greasy dishes: the "tails" burrow into the fat, while the "heads" stay in the water and help the offending substances wash away.
Why do we use milk?
It is the milk's color, not its components, that we need for this experiment. The contrast between the bright colors and the white surface makes this experiment much more spectacular. Any variety of milk — such as coconut, soy or common cow’s milk — is suitable for this experiment!
You can also try using water instead of milk. Compare the brilliance of the colors!
That’s interesting!
What is surface tension?
Picture water molecules as octopuses that are trying to pull each other using all eight limbs at once. In such a scenario, most of these various "pulls" cancel each other out. However, the “octopuses” on the very surface are imbalanced; they are pulled down by their friends below, with no upward pull to counteract this force. Consequently, they are bound together tighter than any other octopuses ⎼ stuck, yet drawn downwards.
Surface tension develops due to this mutual attractive force between molecules. Below the surface, every molecule is attracted to the adjacent molecules on all sides. On the surface, however, the molecules are at a disadvantage: one side is exposed to air molecules. Molecular bonds are all equal in water, yet stronger at the water’s surface because there is no (pulling) attractive force from above.
The stronger the attractive force, the closer the molecules stay together. This results in a “compressed” layer of molecules at the water’s surface, lending that layer distinctive properties. This applies to other liquids as well.
Surface tension is actively exploited by insects known as water striders or water skippers, which can slide along the water’s surface without falling through. This is possible because the force of the water molecules’ mutual attraction is stronger than the force of the push of their legs. Another example: surface tension is strong enough to support a paper clip! Place it carefully on the water’s surface and check it out.
How does the human body digest lipids?
Lipids that we ingest through our food, consist mostly of nearly indigestible molecules. Due to their hydrophobicity (repulsion from water), they stick together and don’t dissolve in the aqueous solution our digestive systems produce. To prevent them from sticking, the liver produces bile (biliary acid). After being treated with bile, the now-isolated lipid molecules are struck by digestive enzymes, which cut them down into small pieces. If we picture enzymes as scissors that cut molecules, fatty acids would be hard to cut. They would stick together and gum up the scissors. However, bile would clean the contaminated scissors and keep them working well. We call this process the emulsification of lipids.
After slicing and enzyme treatment, the resulting fragments are absorbed into our epithelial cells, then the bloodstream, which delivers them to other cells in our bodies, ranging from nervous tissues to the heart. At this point, cells modify lipids on their own, attaching them to proteins or carbohydrates or using them as an energy source. This lipid exchange process resembles a brickyard. First, clay or shale is delivered (food ingestion), then brick is made (emulsifying, slicing, and absorbing) and sent to construction sites where a building is assembled (cells modify lipids freely for different purposes).