Plaster crafts
Add water to solidify!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
If this happens, repeat the experiment. Try to reduce the amount of time you take to mix and transfer the plaster of Paris to the plastic mold.
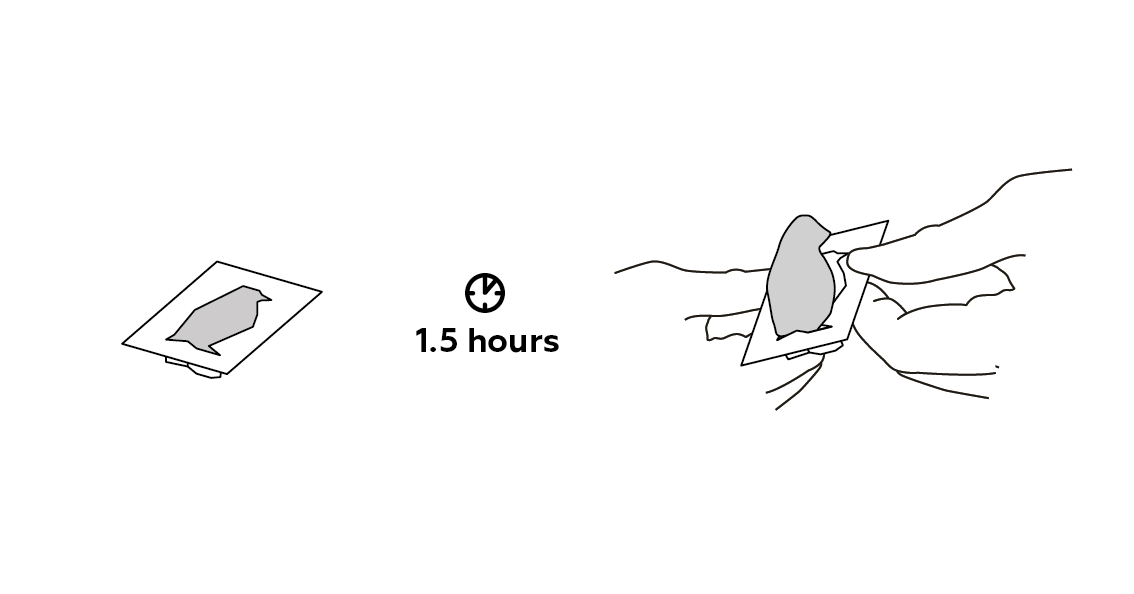
If your plaster of Paris hasn’t hardened after 1.5 hours, then leave it to dry for a little bit longer. The amount of time it takes depends on many things, including the temperature in the room, the humidity, and whether or not the room is drafty.
You can not only keep your penguin – you can color it, too!
Yes, you can! But we recommend washing your hands afterwards.
This does happen sometimes. Use a wooden stick to loosen the reagent in the bottle.
Yes, of course! That’s a great idea! You can use markers or different kinds of paint. But please note that it is better to wait until the plaster of Paris dries completely, which may take a few days. You’ll get the best results if you give it a base coat of white paint first. Then you can paint your penguin however you like!
Step-by-step instructions
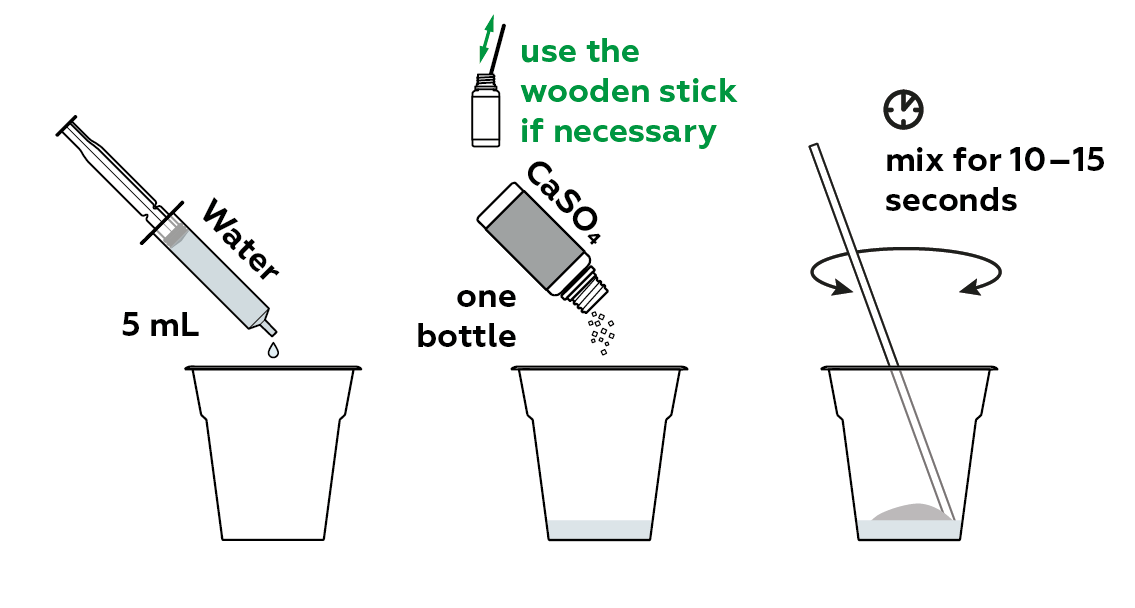
Mix some CaSO4 hemihydrate, also written as CaSO4·0.5H2O, with water.
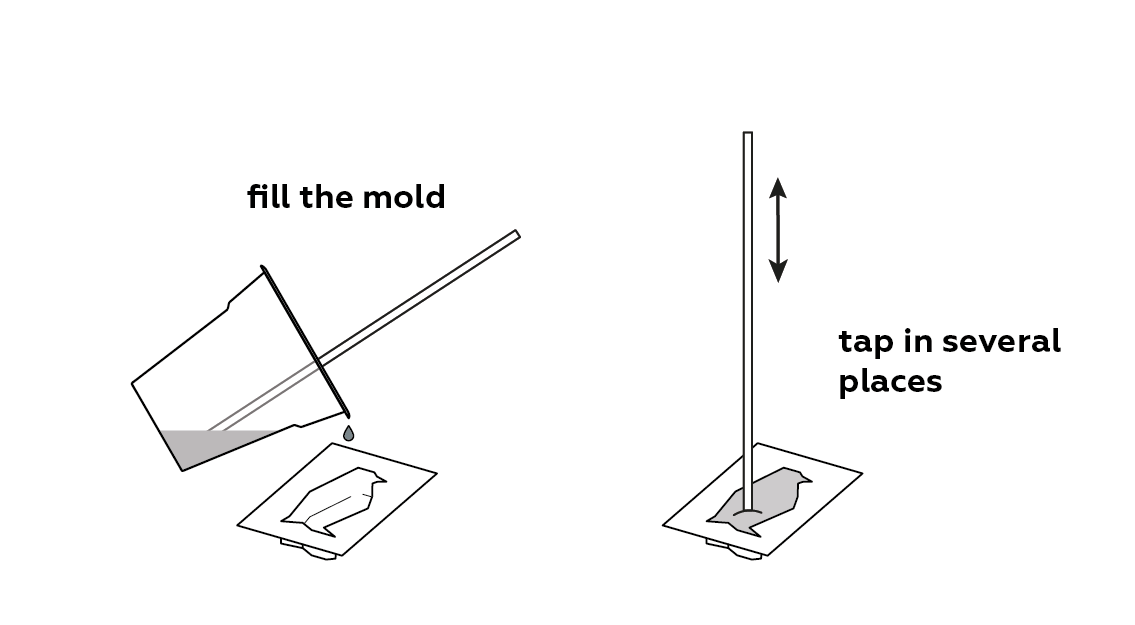
Pour the mixture into a mold.
Let it sit for a while.
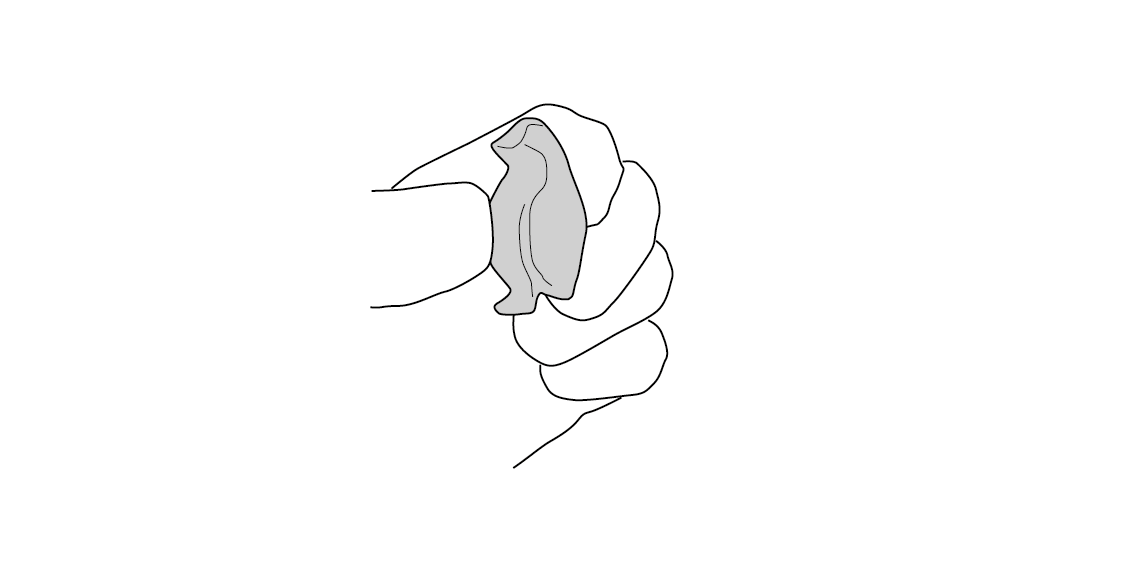
It's solid now! What happened?
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
It's not uncommon for solid chemicals like CaSO4 to have somewhat convoluted formulas, such as CaSO4·0.5H2O. That's because many crystals consist not only of their "main" compound, but also contain some water H2O molecules. CaSO4·0.5H2O, for one, has 0.5 of a water H2O molecule for every CaSO4, or, more simply, one molecule of water for every two CaSO4.
CaSO4·0.5H2O (also known as "plaster of Paris") crystals
are not very soluble in water, and whatever dissolves tends to immediately turn into a solid again. But there's a twist: when CaSO4 has enough water around, it prefers to bind with two water molecules, forming CaSO4·2H2O (known as "gypsum"). These crystals
are very distinct from CaSO4·0.5H2O, and they form separately. CaSO4·2H2O crystals are long and slim, yet tiny, prisms. These prisms form all around the liquid, creating a whole "gypsum thicket." As a result, instead of the thick paste you poured into the mold, you end up with a solid chunk of gypsum.
This property makes calcium sulfate CaSO4 a very handy material for many applications: it can be used to plaster walls, to make surgical casts, or even to manufacture blackboard chalk, which often consists of only about 40% actual chalk CaCO3—the rest is actually gypsum CaSO4.
Follow up
Want to play with your penguin? Use markers to tint it different colors! Now your penguin is ready for all sorts of bright adventures!
That’s interesting!
Are gypsum crystals always tiny?
No, they are not. Gypsum deposits can be found on practically every continent, both as tiny crystals and as masses called gypsum rock. The largest known natural gypsum crystals were found in the Cave of the Crystals in Naica, Mexico. Just think: huge free-standing gypsum crystals that weigh upwards of 55 tons! And New Mexico is home to a large gypsum desert called White Sands, which can even be seen from outer space!