Chemical warmer
Use chemistry to make your own hand warmer!

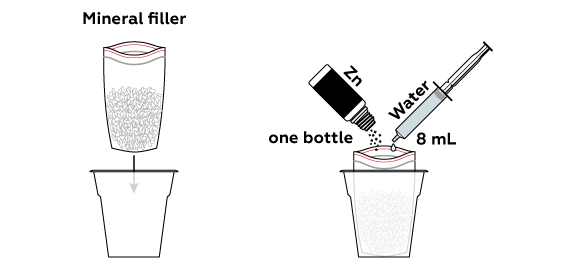
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The filler absorbs excess liquid and helps distribute the reagents evenly in the package.
Add another 3 mL of water to the package and mix the contents thoroughly.
If this happens, just throw the torn bag out and restart the experiment using a new one.
Step-by-step instructions
Since zinc Zn is an active metal, it readily engages in chemical reactions, especially with solutions of compounds of less reactive metals.
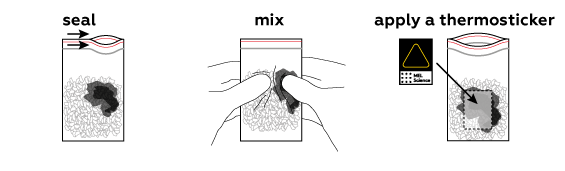
The thermosticker changes color at around 50 °C (about 120 °F).
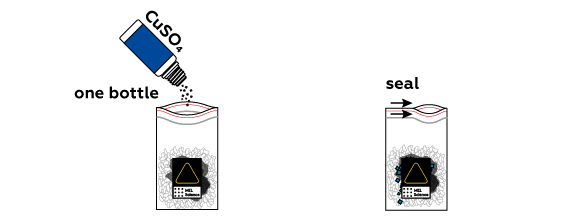
Copper is a less active metal than zinc, so zinc readily reacts with copper sulfate CuSO4 as it dissolves in water.
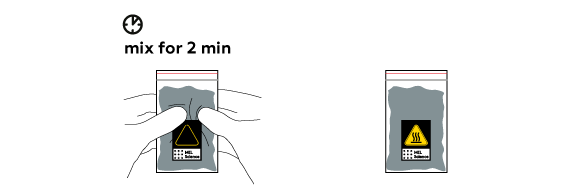
The reaction produces heat that can easily be felt.
Disposal
Dispose of solid waste together with household garbage.
Scientific description
Most chemical reactions have some effect on temperature. This can be obvious, like when something is burning (which means that it is reacting quickly with oxygen). It can also not be obvious at all—especially when a reaction goes slowly, like when rust forms on a piece of iron.
This reaction between CuSO4 and Zn makes copper Cu, ZnSO4, and, well, heat. Much like small crystals of salt dissolve in water faster than large ones, fine Zn powder reacts with CuSO4 much faster than a large piece of Zn would. As a result, the reaction goes quickly enough to produce a lot of heat in a short amount of time.

Interestingly, the very same reaction was used in one of the first electric batteries—a device known as the Daniell cell. Modern batteries use different chemicals, but similar reactions. These reactions can often produce heat along with electricity as well. This is one of the reasons why battery-powered portable devices can heat up when used for a long time.
