How to increase reaction speed
Adding catalyst isn’t the only option
In conducting chemical experiments, experimenters often face the question of how to increase reaction speed, or how to start the reaction, whether it’s a reversible process or not. Here the following methods are required:
- raise the temperature;
- add a catalyst;
- increase the concentration of reagents;
- change the aggregate state of reagents, reduce their size (increase the area of contact of substances).
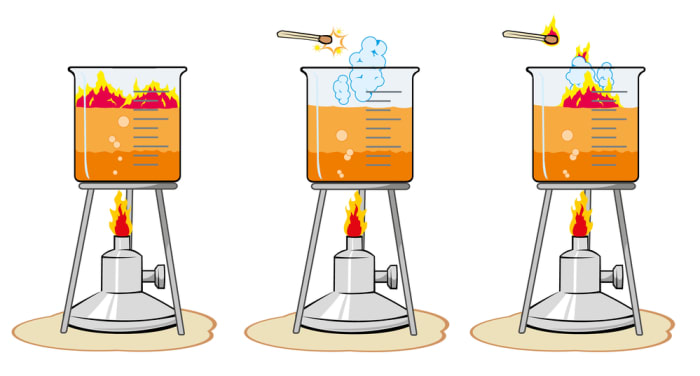
Reaction speed is a characteristic of a chemical reaction which shows a change in concentration of one of the reagents in a unit of time.
Increasing the temperature of the reaction is the most universal and simple method of accelerating the interaction of reagents.
In the interaction of substances with one another, their particles collide, as a result of which the electrons from the outer orbitals may move from one atom to another. Not all interactions lead to a change in the state of mediums, only particles with a high kinetic energy are capable of “knocking out” electrons.
The question of how to increase kinetic energy is solved by heating the substance; the particles increase their movement in mediums, and the number of collisions increases. The reaction accelerates.
To determine the dependence of the speed of the process on the temperature, you must use the Van ‘t Hoff rule, but it cannot work for all types of chemical reactions. It can only be used to give an approximate estimate of the influence of the temperature in a range from 0 to 100 degrees Celsius.
V₂= V₁х
In the home, you can prepare and examine the thermal dependence of a chemical reaction on the example of the interaction of a solution of iodine and starch. This is an interesting experiment which can even be recommended for beginner chemists. Dissolve a teaspoon of starch in a small amount of water, adding a little iodine.
I₂ + (C₆H₁₀O₅)n (starch) ⇄ I₂*(C₆H₁₀O₅)n
The reaction is reversible. The combination obtained as a result is unstable, it colors the water blue, and at high temperature it becomes colorless (the speed of its breakdown increases). When it cools, the water turns blue once again.
Catalyst reactions
In many chemical experiments, to increase reaction speed, you have to add special substances – catalysts. They take part in processes as intermediaries, without becoming part of the final substances, remaining unchanged. At the same time their presence, because of their involvement in the intermediary stages of reaction, helps new substances to appear, speeding up the reaction by many times with a minimum energy loss.
So the presence of a catalyst is like a “side maneuver” in an experiment, when reagent substances do not want to interact or do so too slowly.
A distinction is made between homogenous (when the state, structure of reagents and catalyst are homogenous) or heterogeneous catalysts. Reactions of solid substances are always heterogeneous.
You can see an example of a heterogeneous reaction at home by burning sugar (without a catalyst a sugar cube simply caramelizes). To make the experiment, cover the sugar cube in ash (this is the catalyst in this case) and set fire to it.
С₁₂Н₂₂О₁₁(sugar) + 12О₂ → 12СО₂ + 11Н₂О
For each reaction, different types of catalysts must be used:
- acid;
- alkali;
- metal oxides;
- gases;
- complex organic compounds (many catalyst reactions take place in our body, and the catalysts are called enzymes);
- water;
- sand.
Here you can see detailed instruction on conducting this experiment.
Create an effect of accelerated reaction with change of concentration
You can make a reaction take place more quickly without catalysts and without changing the temperature. A high concentration of substances is used. Let’s see why this method works:
- the distance between particles decreases;
- a greater number of effective collisions between them takes place;
- this leads to an acceleration in the reaction, and a creation of new compounds.
The discovery of the theory of active collisions helped to explain the secret of a change in concentrations.
Look at the example of this reaction:
4НВr + O₂ = 2Н₂О + 2Вr₂
It takes place quite quickly at a temperature of over 4,000, and a similar result can be achieved by increasing the concentration of any of the reagents.