Electricity vs. Iron
Watch as electricity dismantles an iron strip!
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Observe safety precautions when working with batteries.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Ordinary table salt is a substance that belongs to a class of compounds called electrolytes. Upon dissolving, such compounds produce positively- and negatively-charged ions. This makes the solution capable of conducting electricity.
But why is this important? It's quite tricky to explain, but if you want answers, we’ll do our best!
Electrons can move freely in metals – in fact, their movement in a certain direction in an electrical circuit is what creates electric current. However, they cannot swim through a solution on their own. But why do they need to move through the solution at all?
The batteries drain electrons from one strip of iron and transfer them to the other. When a significant lack of electrons arises on the first, and a large excess on the other, the reaction slows down. And the most logical way to restore the balance of electrons is to organize their "transfer" from the area where they are in abundance to that where they are lacking.
This is where the Na+ and Cl- ions that make up salt come to the rescue! Each Cl- ion contains an extra electron, and each Na+ ion is missing an electron. Thus, Сl- ions behave analogously to cargo ships, carrying electrons to the area where they are lacking, while Na+ ions float in the opposite direction, like empty ships.
Interestingly, this process allows you to close the circuit; that is, you can force the charged particles in it to go round in circles. Thus, electrons move through the battery, wires, and strips, while negatively-charged ions, i.e. carriers of electrons, move through the solution from one strip to another.
Inside, a battery is divided into two sections, separated by wadding: one section contains a substance that can give its electrons away, and the other contains a substance that is willing to take them. Pretty much like the magnesium and iron from the Rust protection experiment in this very kit! When the battery isn’t connected to anything, these isolated substances cannot react with each other, but when the electric circuit is closed, the electrons start migrating through it, inducing the beginning of a reaction. Such a reaction is called an oxidation-reduction or redox reaction as, in the process, one substance loses electrons (is oxidized) and the other gains electrons (is reduced).
You can study the principles of how batteries work in more detail and even create your own with the help of the Chemistry & electricity and Zinc-carbon battery kits.
Step-by-step instructions
First, prepare an electrolyte solution with ordinary table salt NaCl. It will accelerate the decomposition of iron Fe. Then, add sodium ascorbate, which will visualize the traces of decomposed iron in the solution.
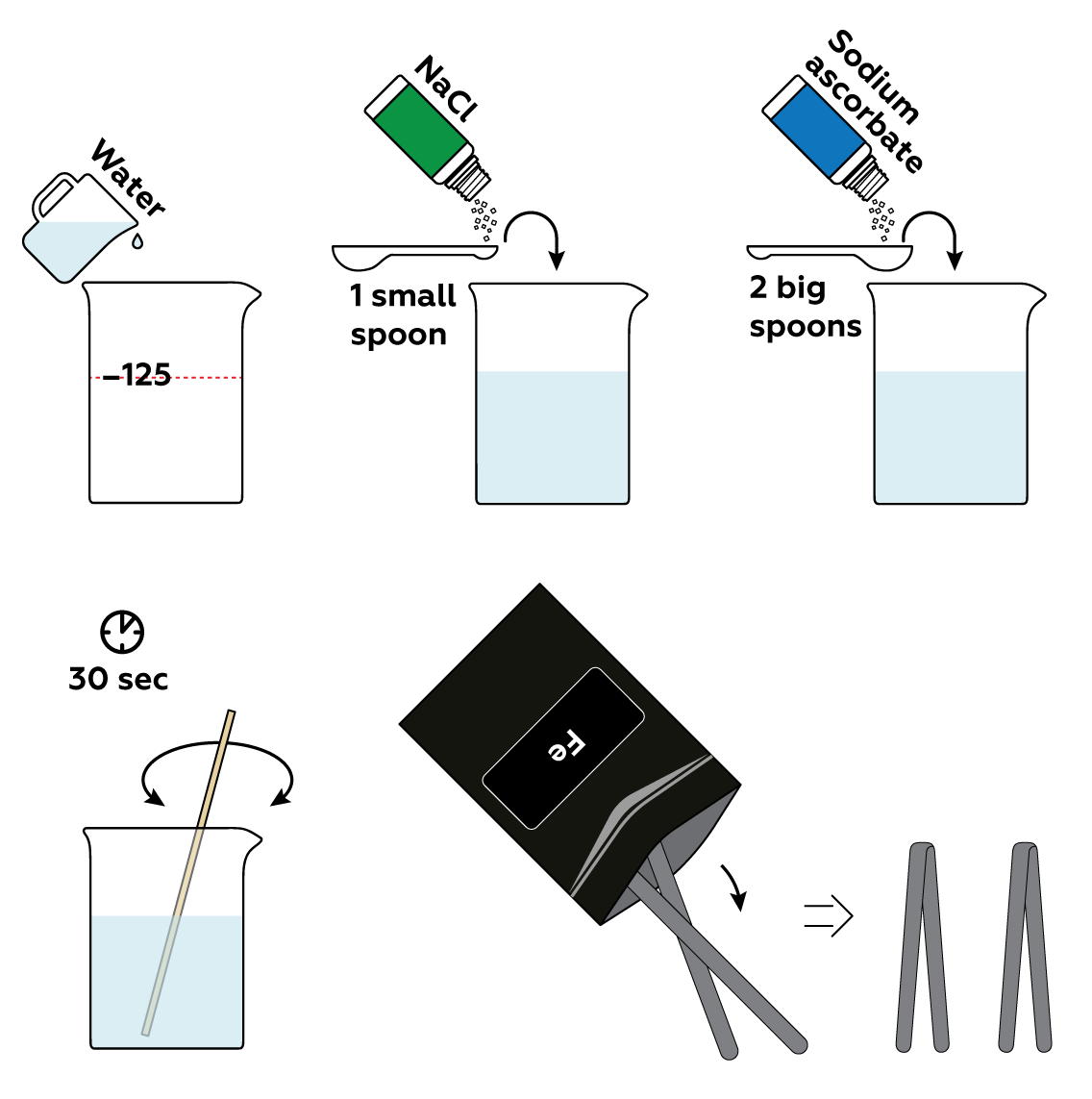
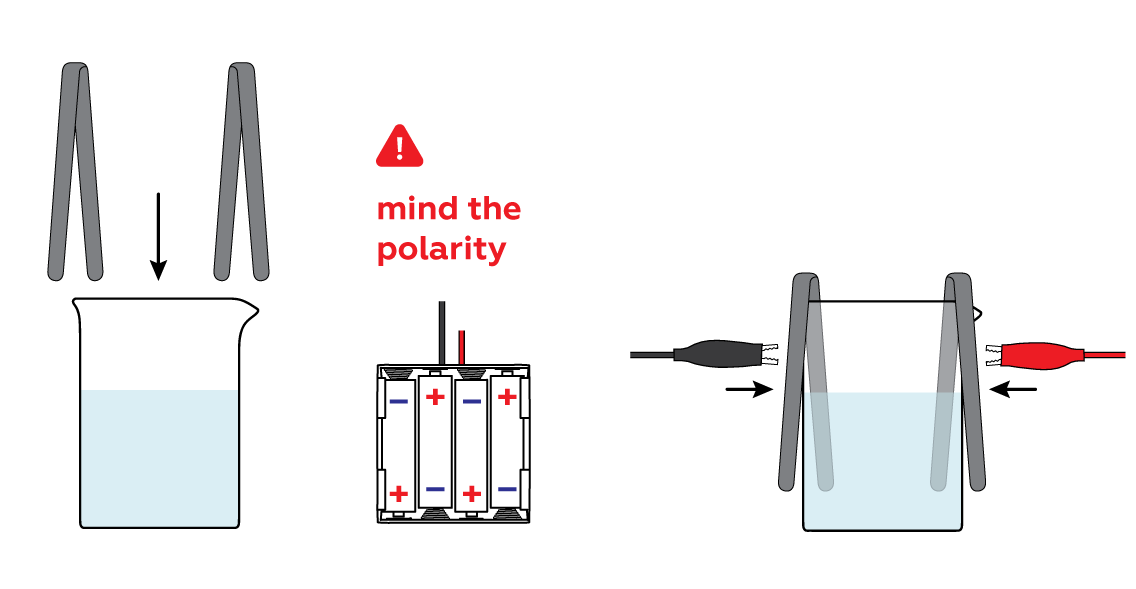
Immerse the iron strips in the solution and connect them to the batteries via crocodile clips.
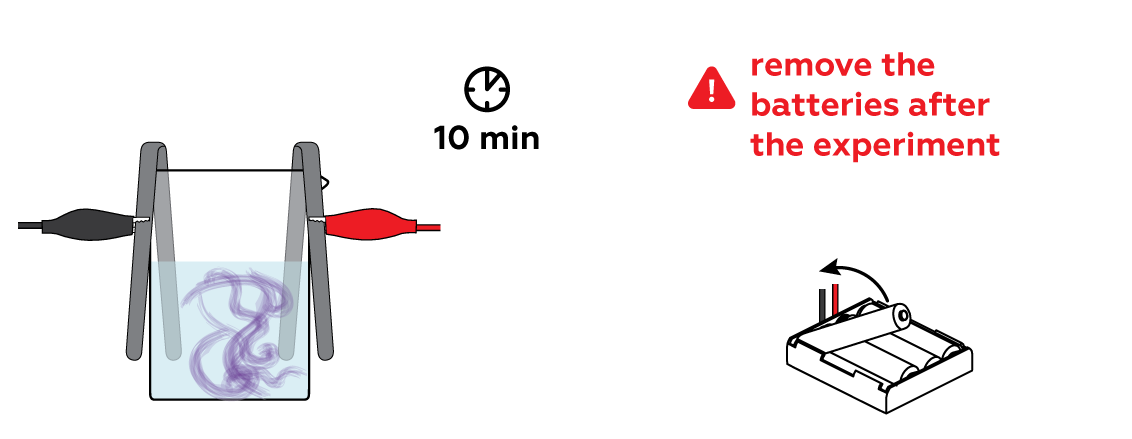
Wow! Look at the purple "fog" materializing near one of the iron strips! These are ascorbate ions detecting traces of decomposed iron. But why is only one iron strip affected?
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description

and atmospheric oxygen O2
. Oxygen and water grab electrons from smooth, shiny iron, turning it into a crumbly, porous, orange-brown substance.

acts like an electron pump that transfers electrons from its "+"










.
What happens with the second iron strip?
While the violet iron ascorbate forms around one of the iron strips, another strip is covered with bubbles. Why is the process on the second strip different from the first?



in the water molecules H2O
from the surrounding solution are eager to take some electrons, pair up into H2

That’s interesting!
Jailbreak with help of ... sauce!
Legend has it that a determined prisoner managed to escape from his cell by dissolving the cell bars using salsa sauce and a radio power supply. This system is similar to the one in our experiment: the sauce contains salts, so it acts like salt water, the radio power supply is a source of current analogous to our battery, and the cell bars dissolve like our iron strip.You can check if this legend is grounded in reality: try to dissolve an iron nail in a sauce of your choice with the help of a battery! Check it out – soy sauce will work perfectly for this experiment!