The reaction between sodium and chlorine
How family ties calmed down two boisterous natures

Despite the first part of the title, our tale will not be about tedious chemical substances and their interactions, our story is about passionate love, and has a happy ending – the formation of a close-knit family.
In our story, we will attend a wedding, observe the meeting and development of relations between sodium and chlorine, two lovers with very difficult characters, watch a fireworks display, visit the shore of the Dead Sea, reveal the secret of the solution which saves the lives of people, and much more In chemistry, everything is like in people’s lives: meetings, reunions and partings.
Imagine that we are present at a wedding, with music and flowers: sodium and chlorine have decided to live together. Or to use the language of chemistry, two substances enter into a combination reaction.
First, let’s get to know the couple better.
Sodium: physical and chemical properties
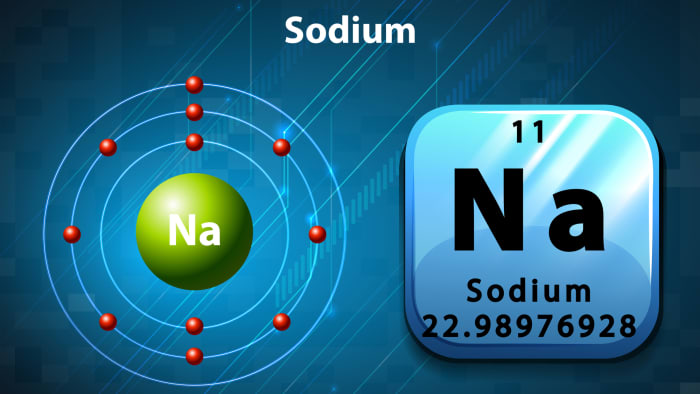
So, let’s meet the groom, “sodium”. Usually the bride’s parents are interested in where the groom is from. And he has a quite definite place of residence in the periodic table – 1st group, atomic number 11. He is from the group of alkaline metals.
Sodium is a simple substance, a silvery white metal that is light and soft, oxidizes quickly in the air, and reacts violently with water, with an explosion. As we can see, the groom has a difficult, explosive character.
Additionally, sodium interacts:
- with oxygen;
- with many non-metals (except nitrogen, iodine and noble gases);
- with acids (diluted and concentrated);
- with liquid and gaseous ammonium;
- with mercury;
- with some organic compounds.
Chlorine: physical and chemical properties

Who’s the bride, then?
Chlorine is an element in the 3rd period of the VII A group, with an atomic number of 17.
This is a simple substance, a non-metal in the group of halogens, a yellow-green poisonous gas with a harsh suffocating smell, it is thermally stable, does not burn in air, and when mixed with hydrogen explodes in light.
Besides hydrogen, chlorine:
- interacts with non-metals;
- interacts with almost all metals;
- it forces bromine and iodine out of their bonds with hydrogen and metals;
- when dissolved in water or alkalis it forms hypochlorous, perchloric or hydrochloric acid, or their salts;
- with calcium hydroxide, it forms lime chloride.
- interacts with organic substances.
As we can see, the couple have quite boisterous natures. Both sodium and chlorine enter into reactions with different substances and compounds.
Although sodium is a metal, it is soft and malleable: you can cut it with a knife, like butter. Chlorine is not easy-going, either – it’s a poisonous suffocating gas, and was the first poison to be used in war.
Based on this information, you’d think that an alliance between these two would be simply horrendous.
But let’s not jump to conclusions. Let’s see what the reaction of sodium and chorine is like, and how they interact.
2Na + Cl₂ = 2NaCl + Q
As we can see, as a result of the reaction of such seemingly “restless” substances (explosive, and chlorine is also poisonous), a compound is created that is quite peaceful, safe and even edible – sodium chloride (kitchen salt).

Visually, this reaction of sodium and chlorine resembles a fireworks display. In a flask full of chlorine, small pieces of sodium are placed – there are flashes of light, flame, and then thick white smoke! It’s quite spectacular! But this white smoke is in fact tiny crystals of kitchen salt. This is how passions flare up between our lovers! It’s more intense than a Mexican soap opera!
Now we’ll describe the reaction of sodium and chlorine from different standpoints.
- On the one hand, this is a combination reaction. One simple substance bonds with another simple substance, and a compound forms.
- From the energy standpoint, the reaction is exothermic, as it takes place with the release of energy – light and heat (in the amount of 819 kJ).
- By its aggregate state, it is heterogenic, i.e. a solid substance enters into a reaction with gas, as a result of which a solid substance forms.
- It is an irreversible reaction, as it proceeds to its completion with the formation of a stable product of reaction.
- Furthermore, it is an oxidation-reduction reaction.
Let us look in more detail at the last point, as it explains why exactly sodium reacts with chlorine. Let us define what an oxidation-reduction reaction is. Reactions accompanied by the transfer of electrons from one atom to another are called oxidation-reduction reactions.
The oxidizer is the atom which accepts the electrons in the reaction. The reducer is the one that gives up the electrons.
When sodium and chlorine interact, the reaction is accompanied by the transfer of electrons. Electrons in the chemical world are like money in people’s lives. Some have a lot, some have little, some steal it, some lose it. We’ll see whether it’s the bride or groom doing the stealing in this case.
The electronic structure of sodium and chlorine atoms
As the atomic number of the element in the periodic table determines the charge of its nucleus, and accordingly its number of electrons, let’s examine the electronic structure of sodium and chlorine, or figuratively speaking, the property of the bride and groom.
Na +11 )1s2 )2s2 2p6 )3s1
CL+17 )1s2)2s22p6)3s23p5
From the electron formula of sodium, we can see that on the outer electron sublevel it has 1 electron, which it can easily give up. For chlorine to reach the p-sublevel, it lacks one electron, which it takes from sodium.
So to continue our story, the bride takes some of the groom’s property, which he gladly shares so they can be together.
We should note that chlorine is one of the strongest oxidizers. Sodium easily enters into a reaction with chlorine, as one element easily gives up an electron, and the other easily takes it. The family is a close-knit one, in the form of a wonderful compound, sodium chloride. We can hardly do without kitchen salt, after all – it is indispensable in cooking; it is used in medicine in solutions for removing swelling; in municipal services as an anti-freeze agent; to soften water; and sodium chloride is also used in the chemical industry.
Incidentally, the well-known saline solution that has saved many people’s lives is a 0.9% aqueous solution of sodium chloride. Click here to see easy experiments with kitchen salt.
Kitchen salt is produced by evaporating saline solutions. The world leader for the production of kitchen salt is China. It is found in nature in the form of deposits of halite and sylvinite, brines of salt lakes, and mineral mixtures in seas. It usually takes the form of white crystals, but natural salt deposits can also be blue, yellow, grey, or even red.
Let’s make a mental shift to the Dead Sea.

We don’t drown in it because of the high concentration of salts dissolved in it (35 g per 1 liter of water), including sodium chloride.
So, to sum up: the violent, unpredictable and sometimes poisonous sodium and chlorine have joined together; the reaction has given us the harmless and even useful compound of kitchen salt. As we said originally, family ties have calmed down two boisterous natures and made them happy and harmless to others. This is the happy end of our story.