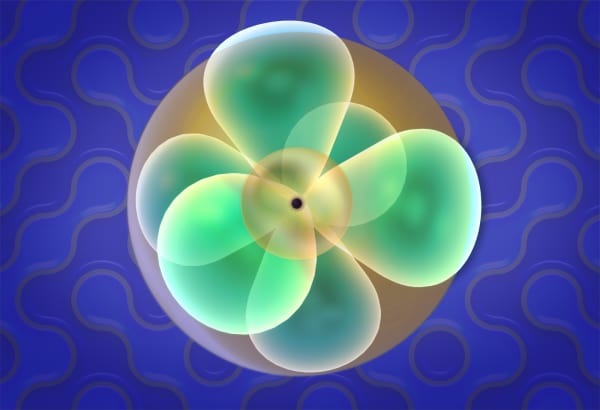
Make your atom
An interactive lab where students can assemble any atom they want, and see what this element will look like.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript















Teacher's notes
Keywords
atom, nucleus, proton, neutron, isotope, electron, electron orbital, electron configuration diagram, s-orbital, p-orbital
Students will
- Assemble any atom they choose
- Review electron orbital names, shapes, and order of appearance
- Review electron configuration diagrams
- Assess their isotope’s stability
Lab description
We recommend using the ‘Make your atom’ lab to not only review atomic structure and electron configuration, but as a powerful tool to demonstrate how electron structure affects elemental chemical properties. In addition to an electron configuration diagram and 3D visualization of orbitals, this lab provides information about the atom, such as its size and select characteristics.
The 3D visualization of electron orbitals appears for each electron as soon as it is added. To the right of the 3D visualization, the electron configuration diagram is filled. Highlighting the electron orbital on the 3D model highlights the corresponding orbital on the diagram and vice versa.
The lab draws upon a comprehensive database of elements, including all known isotopes. After the nucleus is completed, information about the isotope (stability and half-life, history of discovery) appears to the left of the 3D visualization.
The lab serves primarily as a skill builder for electron configuration, although it can be used in a variety of topics throughout the curriculum, such as:
- Periodicity and properties
- Ion formation
- Bonding
- Stability of isotopes
- Radioactivity and half-life
Topics to discuss
- Electroneutrality of the atom
- Aufbau principle, Pauli exclusion, & Hund's rule
- Energy levels
- Isotope stability and half-life