Chemical worms
Turn two liquids into wiggly worms!
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Sure! Just repeat the experiment using red glitter instead of blue. You can also try adding other dyes – or not adding any at all.
Sodium alginate is a substance of natural origin. It's made of alginic acid, which is found in brown algae.
The blue worms look pretty unappetizing, and for good reason! You should never eat or even taste chemicals that are not intended to be eaten, such as the ones provided in chemistry sets! That being said, a very similar reaction is used fairly often in a style of cuisine called "molecular gastronomy." The main difference is that instead of the calcium chloride CaCl2 you used in the experiment, molecular gastronomists normally use calcium lactate and add food colorings and flavorings to the mixture. The resulting squishy threads and beads provide interesting textures for many dishes in this style.
Step-by-step instructions
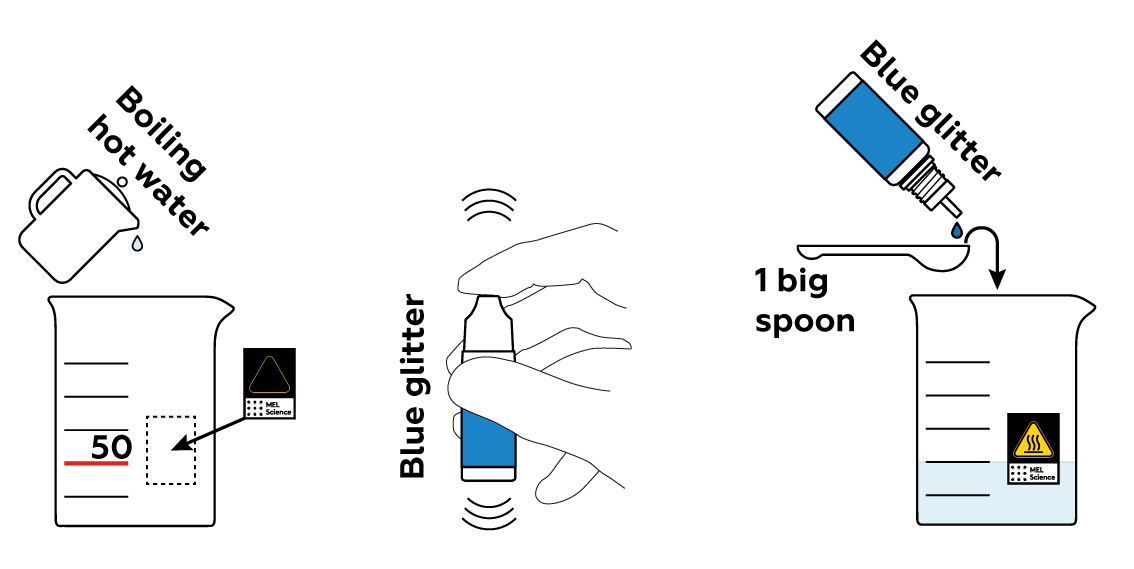
To make your crafts colorful and shiny, add some blue glitter to hot water.
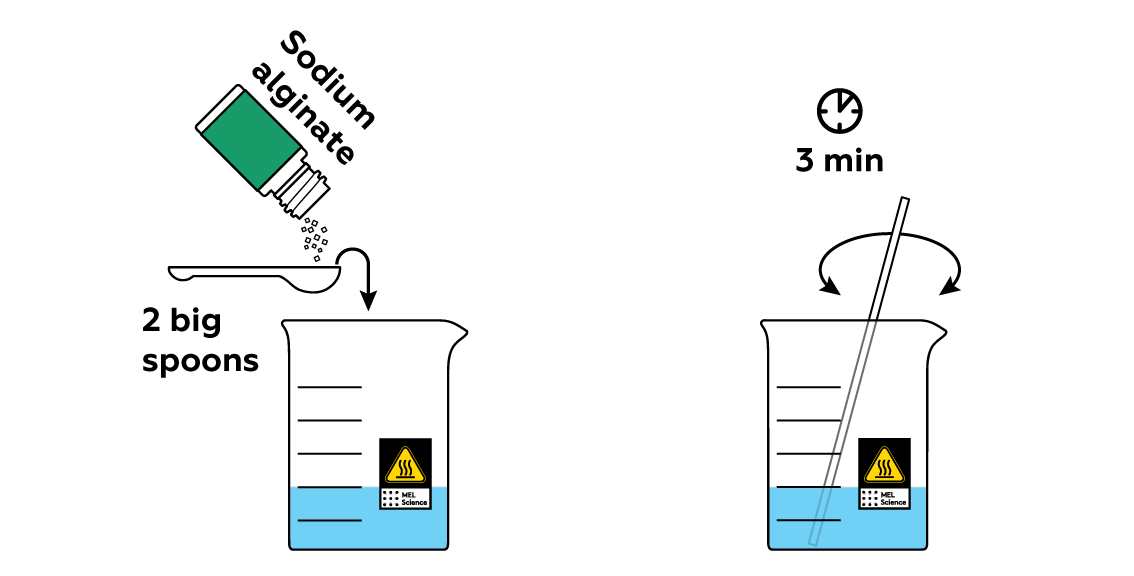
Add some sodium alginate to the solution.
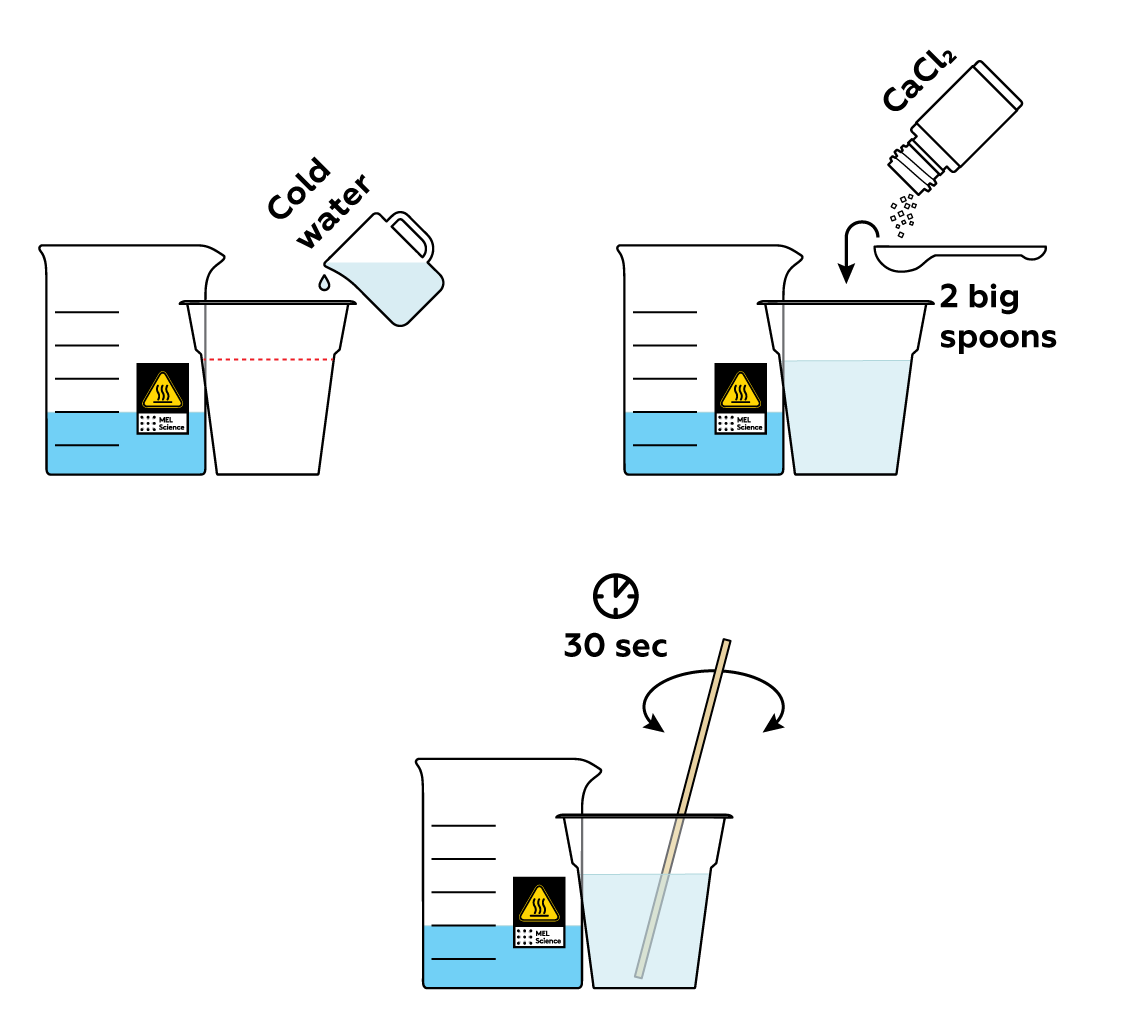
Prepare some calcium chloride CaCl2 solution in a cup.
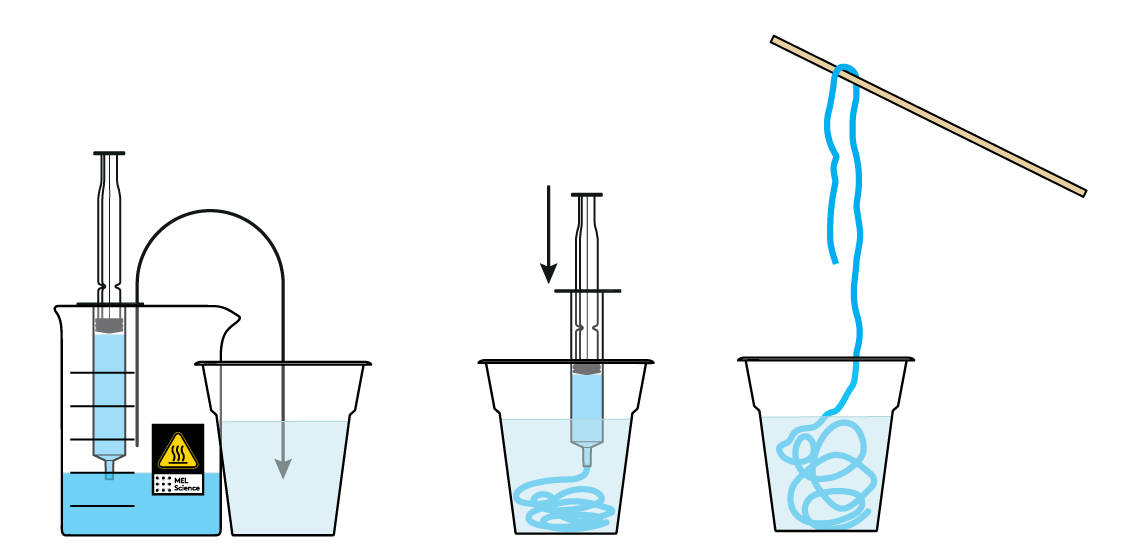
Draw the blue mass into a syringe and squeeze it into the CaCl2 solution. What a cute sparkly worm you've made!
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description

. Like any long threads, they can get tangled—that's why the sodium alginate solution is so viscous. As soon as you squeeze the sodium alginate from the syringe into the CaCl2 solution, Ca2+ ions
from the solution come into contact with the outermost alginate ions and weave them together like threads in a fabric
. As a result, the “worm” is covered with a dense shell, but remains liquid on the inside.
That’s interesting!
What are alginates used for?
Alginic acid and its salts—alginates—are naturally present in the cell walls of brown algae. Despite their marine origin, these substances have established themselves as essential substances for land-dwellers.
Due to their ability to increase the viscosity of liquids, alginates are widely used in the food industry as thickening or gelling agents. They are added to ice cream to maintain its shape and prevent the formation of sugar crystals in it, as well as to mayonnaise to avoid its separation into water and oil. Alginates also have some pharmaceutical applications: they are added to tablets as binders that hold the medicinal component together. Alginates come in handy even in cosmetics: they help sustain lipstick’s vibrance by forming a thin film on the surface of the lips.