Periodic table box
You will learn that each box of the periodic table contains a lot of information about the element: name and symbol of the element, number of protons and electrons, atomic mass, and electron configuration.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

In this lesson, we will tell you about the information on each element that you can find in the periodic table.

Let's click on an element, for example, sulfur. You see the square, where sulfur is? This is the symbol of the element.
This is the name of the element. Sulfur. This is the atomic number of sulfur. 16. It shows the number of protons in the nucleus of a sulfur atom.

Question: how many electrons are there in a sulfur atom?
Yes. The number of electrons in an atom is equal to the number of protons in the nucleus of the atom.

This is the average atomic mass of the sulfur atom. It is the weighted average mass of the isotopes of sulfur in a naturally occurring sample of sulfur.
In the lesson "atomic mass" we will show you how to calculate it.

Now, look at the right panel.
Here you see the electron configuration of the sulfur atom. It is also shown in the bottom of the square in short form.

Look at it carefully.
Sulfur has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital and four electrons in the 3p orbital.
So, we have one s two, two s two, two p six, three s two, three p four.

The sum of superscripts equals the number of electrons in the atom.
For sulfur it is 16.
Sometimes we can write the electron configuration in shorthand. For sulfur it looks like this.
Neon in brackets means that sulfur has filled first and second levels, like the neon atom has. Also sulfur has 2 electrons in the 3s orbital and four electrons in the 3p orbital.

So, we write Neon in square brackets, and after that write three s two, three p four.
Now, you see, that sulfur has 6 valence electrons. This is the main information about the element that you can find in the periodic table.
Also, the color of the square in different tables usually means something. In our table, the pale blue color of sulfur square means, that sulfur is a nonmetal.
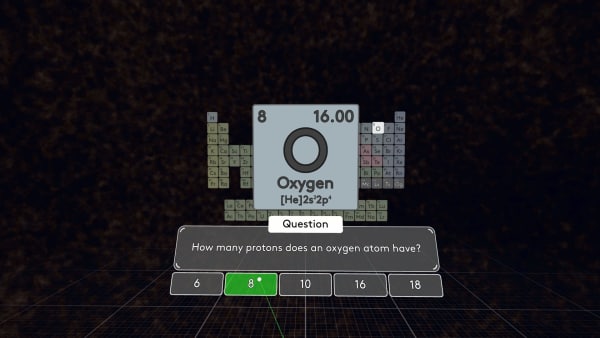
Let's take another element, for example, oxygen.
How many protons does oxygen have? Yes, an oxygen atom has 8 protons.
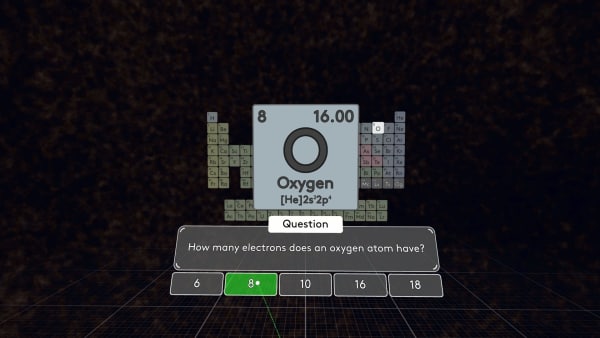
How many electrons does oxygen have? You are right. In an atom, the number of electrons is equal to the number of protons.

How many valence electrons does oxygen have?
Valenсe electrons are the electrons located on the outer electron shell of an atom. There are 2 electrons on the 2s orbital and 4 electrons on the 2p orbital. So, the oxygen atom has six valence electrons.
Now if you want to know some information about other elements, you should go to the Lab Periodic table.