Water hardness
Find out the difference between permanent and temporary hardness of water


Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the tray.
- Place the stove on the cork hot pot stand. Do not touch the stove after the experiment - wait until it cools down.
- Remove protective gloves before lighting the candle.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Solution in the flask doesn’t get cloudy after step 6.
Probably, the solution in the flask hasn’t yet heated up enough. Wait for 5 min. longer.
How to use a thermosticker?
Attach the sticker onto the flask or the stove. At a temperature around 60–70 oС, the triangle turns from black to yellow to warn that an object it’s been attached to is hot and shouldn’t be touched! You may only pick up or touch the flask when it cools down and the triangle turns black again.
Step-by-step instructions
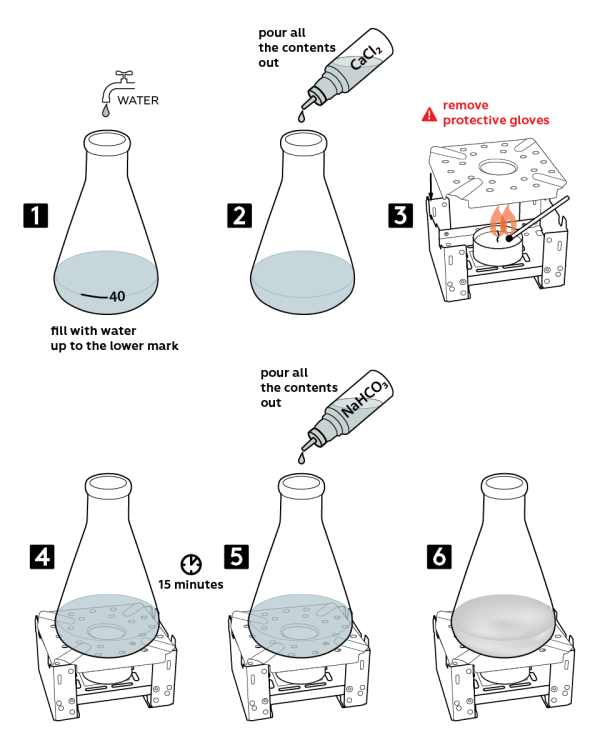
- Pour water into a flask up to a "40" mark.
- Add there all the 0.2M calcium chloride CaCl2 solution from a vial.
- Take a fuel tablet stove and place a candle onto it. Remove protective gloves and light the candle. Set a flame diffuser onto the stove, as shown.
- Put the flask onto the flame diffuser. Wait 15 min.
- Pour all the 0.3M sodium hydrocarbonate NaHCO3 solution from a vial.
- Water in the flask will turn cloudy.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash off with excess of water.
Scientific description
What is water hardness?
Water hardness is a value that reflects the amount of dissolved calcium, magnesium, and iron salts in water. There is temporary hardness (that can be removed) and permanent hardness. Temporary hardness is caused by calcium and magnesium bicarbonates (Ca(HCO3)2 and Mg(HCO3)2), and permanent harness – by their sulfates ((CaSO4 and MgSO4) and chlorides (CaCl2 and MgCl2).
Thus, hard water is water that contains a lot of calcium, magnesium, and iron salts simultaneously.
Why do we add СаCl2?
By adding calcium chloride CaCl2 into water, we artificially increase its hardness. As mentioned above, CaCl2 causes permanent (i.e. unremovable by boiling) water hardness. And the first part of the experiment demonstrated this fact: with boiling, no noticeable precipitation occurs on the walls.
What happens when we add NaHCO3?
Adding sodium bicarbonate NaHCO3 yields formation of calcium bicarbonate in the solution:
2NaHCO3 + СаCl2 ↔ Ca(HCO3)2 + 2NaCl
And due to formation of Ca(HCO3)2, hardness of our water becomes temporary – now, it can be removed by boiling.
What happens upon water heating?
When we heat water, fairly soluble calcium bicarbonate turns into poorly soluble carbonate:
Ca(HCO3)2 → CaCO3↓ + CO2↑ + H2O
White coating observed on the bottom and walls of the flask is calcium carbonate.
Why is scale formed, and how to remove it?
Scale (or limescale) is insoluble calcium carbonate CaCO3 that precipitated during thermal decomposition of calcium bicarbonate Ca(HCO3)2:
Ca(HCO3)2 → CaCO3↓ + CO2↑ + H2O
Even though a thick grey layer of scale doesn’t make dishes look any prettier, it brings no harm. Moreover, it can remove excessive hardness of tap water. Besides, scale can be easily removed from kettles and pots by cleaning them with citric acid С6H8O7 solution:
3CaCO3 + 2С6H8O7 → Ca3(C6H5O7)2 + 3CO2 + 3H2O
The reaction produces calcium citrate Ca3(C6H5O7)2 that is well soluble in water.
Dirty beige color of scale is due to presence of iron oxide Fe2O3. Tap water contains some amount of iron in form of Fe2+ ions, but upon boiling it precipitates out.
Interestingly, scale has the same chemical composition as natural limescale. Sometimes, they find whole mountains formed out of this mineral. Usually, such massive natural formations feature numerous caves – speleologists’ favorite! Those caves are easily formed in limestone: even slightly acidic natural water dissolves carbonates on its way, thus, forming curious passages in the mineral.
In addition, calcium carbonate is the main component of regular chalk used to write on a blackboard or draw on asphalt. Moreover, chalk is employed as a food additive – a white food color, E170.
Recall that calcium carbonate is the substance that makes an eggshell hard. There is an experiment in the MEL Chemistry set “Chemistry of food,” in which calcium carbonate is dissolved with regular vinegar (see experiment “Rubber egg”).
How to soften water?
Water containing low amounts of calcium and magnesium salts is called soft. And the process of removing water hardness is softening.
The easiest way to soften water, as it is demonstrated in our experiment, is boiling. Upon heating, calcium and magnesium bicarbonates (Ca(HCO3)2 and Mg(HCO3)2) undergo thermal decomposition:
Ca(HCO3)2 → CaCO3↓ + CO2↑ + H2O
Mg(HCO3)2 → MgCO3 + CO2↑ + H2O
MgCO3 + H2O → Mg(OH)2↓ + CO2↑
Boiling (thermal softening) is the most ancient method to soften water for domestic needs. Of course, this process only removes temporary (carbonate) hardness. Permanent hardness persists, as we can observe in the first part of our experiment: water saturated with calcium chloride CaCl2 leaves no precipitate upon boiling.
Distillation is closely connected with boiling. During distillation, evaporated liquid is consequently condensed on a cooled surface and, thus, is collected in form of drops. Water purified via such process is called distilled water and doesn’t contain any metal ions. Because of low mineralization, distilled water is not suitable for drinking, as it “washes out” minerals from the body. However, distilled water is widely used in science and industry.
Below you may find more information about modern methods for water softening.
Obviously, boiling is one of the easiest ways to soften water. However, such process has significant disadvantages – namely, low efficiency and high energy consumption.
Another way to soften water is to use reagents. They transfer magnesium and calcium ions into insoluble form by adding certain chemical substances – for example, calcium hydroxide Ca(OH)2 (the process is called lime softening):
Ca(OH)2 + Ca(HCO3)2 → 2CaCO3↓ + 2H2O
Mg(HCO3)2 + 2Ca(OH)2 → Mg(OH)2↓ + 2CaCO3 + 2H2O
Similarly to boiling, lime softening only removes carbonate hardness. In order to remove permanent (non-carbonate) hardness, deeper water softening is necessary, so in addition to slaked lime, they use sodium carbonate Na2CO3:
Ca2+ + Na2CO3 → CaCO3↓ + 2Na+
Mg2+ + Na2CO3 → MgCO3↓ + 2Na+
MgCO3 + Ca(OH)2 → Mg(OH)2↓ + CaCO3↓
For an even more effective removal of calcium and magnesium ions from water, they use “big guns” – sodium phosphate Na3PO4:
3Ca2+ + 2Na3PO4 → Ca3(PO4)2↓ + 6Na+
3Mg2+ + 2Na3PO4 → Mg3(PO4)2↓ + 6Na+
A drawback of this water softening method is the necessity to dose the reagents very precisely.
In industry, the most widely used technique for water softening involves ion-exchange resins. Water is passed through a special filter that withholds calcium Ca2+, magnesium Mg2+, iron Fe2+, and manganese Mn2+ ions. These “caught” ions are replaced with potassium K+, sodium Na+ or hydrogen H+ ions released into a solution.
This method is very efficient for water softening. Advantages of this technique are low cost of reagents and absence of complex procedures, such as sedimentation and removal of a precipitate. By the way, this method is exactly how water is softened in a dishwasher. For filter to last longer, they pour salt NaCl in a dishwasher, so that it replaces calcium and magnesium in there and saturates the filter with sodium ions.
In a laboratory, they often use another water treatment process called reverse osmosis. Besides, this method is sometimes even used in homes. Water is passed through a membrane that doesn’t let metal salts go through. Importantly, reverse osmosis can only accept water that has already been preliminarily treated. A membrane is very thin and delicate: it may be damaged with high concentrations of salts. Reverse osmosis is a very expensive water treatment process, but in turn, it is relatively fast and convenient.
That’s interesting!
Which water is better for you: hard or soft?
The answer is simple: everything is good in moderation. An ideal option for everyday household use is medium hard water that contains some amount of calcium, magnesium, iron, and manganese salts. The right balance is always the way to harmony.
Hard water
First of all, let’s find out why it is a problem when water is too hard. Consuming hard tap water on a regular basis may contribute to formation of kidney and bladder stones. There are no other medically proven potential hazards to human health from drinking it. Using hard water for bathing or face washing may often cause skin irritation, especially in children. Further speaking, hard water may cause some inconvenience in household activities: calcium and magnesium salts responsible for water hardness can form insoluble compounds with fatty acids contained in soap. Not only it increases soap consumption — in some regions, due to water hardness, up to 35 % of soap is spent to soften water, and then only 65 % is used for hygienic purposes. Sadly, it also leads to precipitation of insoluble calcium and magnesium salts (stearates) in sewer lines, thus, settling there soap scum. Today, most detergents, unlike regular soap, contain synthetic additives that do not react with hardness salts. Hence, nowadays the “soap problem” of hard water is passing away. Unfortunately, another problem arose: in hard water, laundry machines and dishwashers heating elements gradually deposit a layer of limescale and eventually stop working because of that. In a household, hard water causes some inconvenience, but on an industrial scale, it can create a real trouble. Even a millimeter-thick layer of limescale deposited on inside of equipment dramatically lowers its heat exchange efficiency. Not only it increases consumption of electricity for a process itself, but it can also damage the equipment. Imagine a huge kettle that consistently grows a thick layer of limescale on the bottom. Sooner or later, it will fail! And in industry, they use setups and instruments much more sophisticated than a kettle. Thus, both heating and cooling systems require careful consideration of supplied water.
Processes involving chemicals also demand thorough water treatment. For instance, textile dying is only possible with the use of extra soft water because some dyes may form insoluble compounds with calcium, magnesium, manganese, and iron ions. With hard water, that process with certain dyes could be very complicated.
Soft water
Now, let’s consider advantages and drawbacks of using soft water.
Consuming soft water on a regular basis may cause issues with teeth and bones, as with drinking water we usually receive a considerable amount of necessary minerals. Luckily, the problem isn’t that huge, since the need in calcium, magnesium, and manganese consumption may be satisfied with healthy nutrition.
In soft water, soap foams much more effectively than in hard water. However, it takes considerably more soft water to rinse off a detergent than it does with hard water.
Moreover, water piping suffers from extra soft water: upon heating, water with low concentration of salts may gradually dissolve metal constructions. In chemical industry, they often use water that is more than just softened — it is distilled. Not only distilled water is very soft — it is also free of numerous impurities that do not at all affect water hardness, such as sulfates (SO4)2-, chlorides Cl-, and sodium Na+ and potassium K+ ions.
Distilled water: can’t get any softer
Distilled water is water that has been purified via a complex process called distillation. Distilled water contains almost no impurities. Interestingly, distilled water can be overcooled below the freezing point or overheated above the boiling point! Distilled water is normally used in chemical laboratories and in chemical industry where purity of prepared solutions is very critical. Besides that, distilled water is employed in food processing (to create certain drinks), in automotive industry (to prepare electrolyte for batteries), in medicine and color printing.
There is also water that has been purified via distillation twice — so-called double-distilled, or bidistilled, water. In composition, it is very close to chemically pure water. Is there a method to obtain water of even higher purity? Producing such water requires use of gamma-radiation followed by bubbling with inert gas — argon Ar.
Indeed, regular water contains many impurities invisible to the eye: salts, dust particles, minerals, and organic compounds. A complete opposite of regular water would be chemically pure water that contains only water H2O molecules.