Igniting iron
So you think iron doesn’t burn?
Reagents
Safety
- Put protective eyewear on.
- Conduct the experiment on the plastic tray.
- Keep a bowl of water nearby during the experiment.
- Keep flammable materials and hair away from fire.
- Place the stove on the cork hot pot stand. Do not touch the stove after the experiment – wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Not a problem! Just gently arrange the pieces so that they’re touching one another.
The problem is likely that oxygen can’t access the iron well enough to support combustion. Fluff the iron wool again and try to light it one more time.
Be sure to wait till everything cools down and the thermosticker turns black. The iron wool is perfectly safe, so you can dispose of it with ordinary household waste.
Step-by-step instructions
This experiment looks better in the dark.
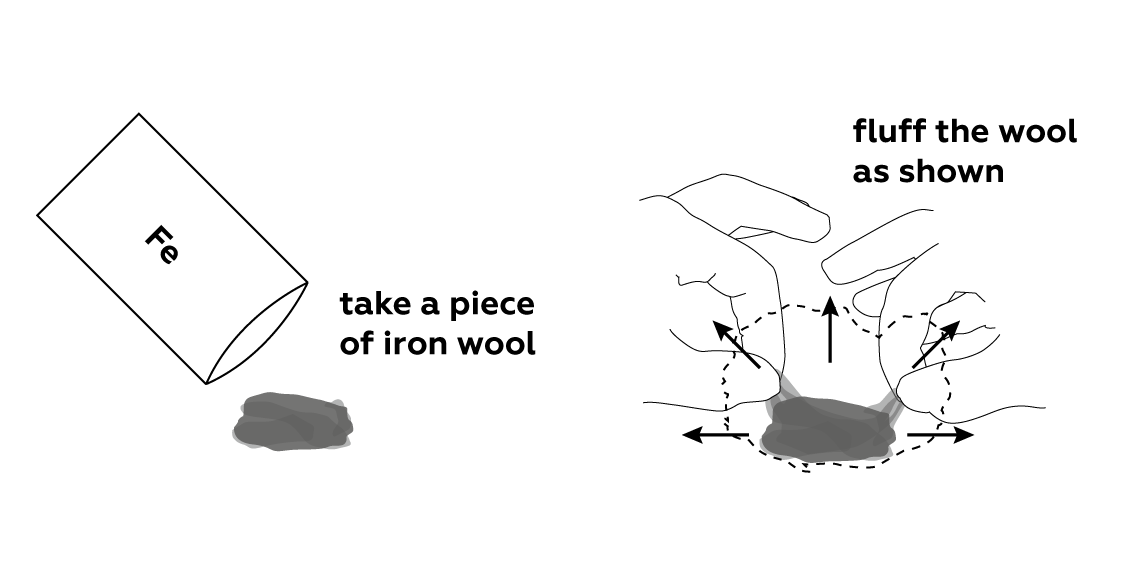
Steel wool, also known as iron wool, is made of fine iron threads. Fluff the wool up a little bit to make it easier for oxygen to access the iron.
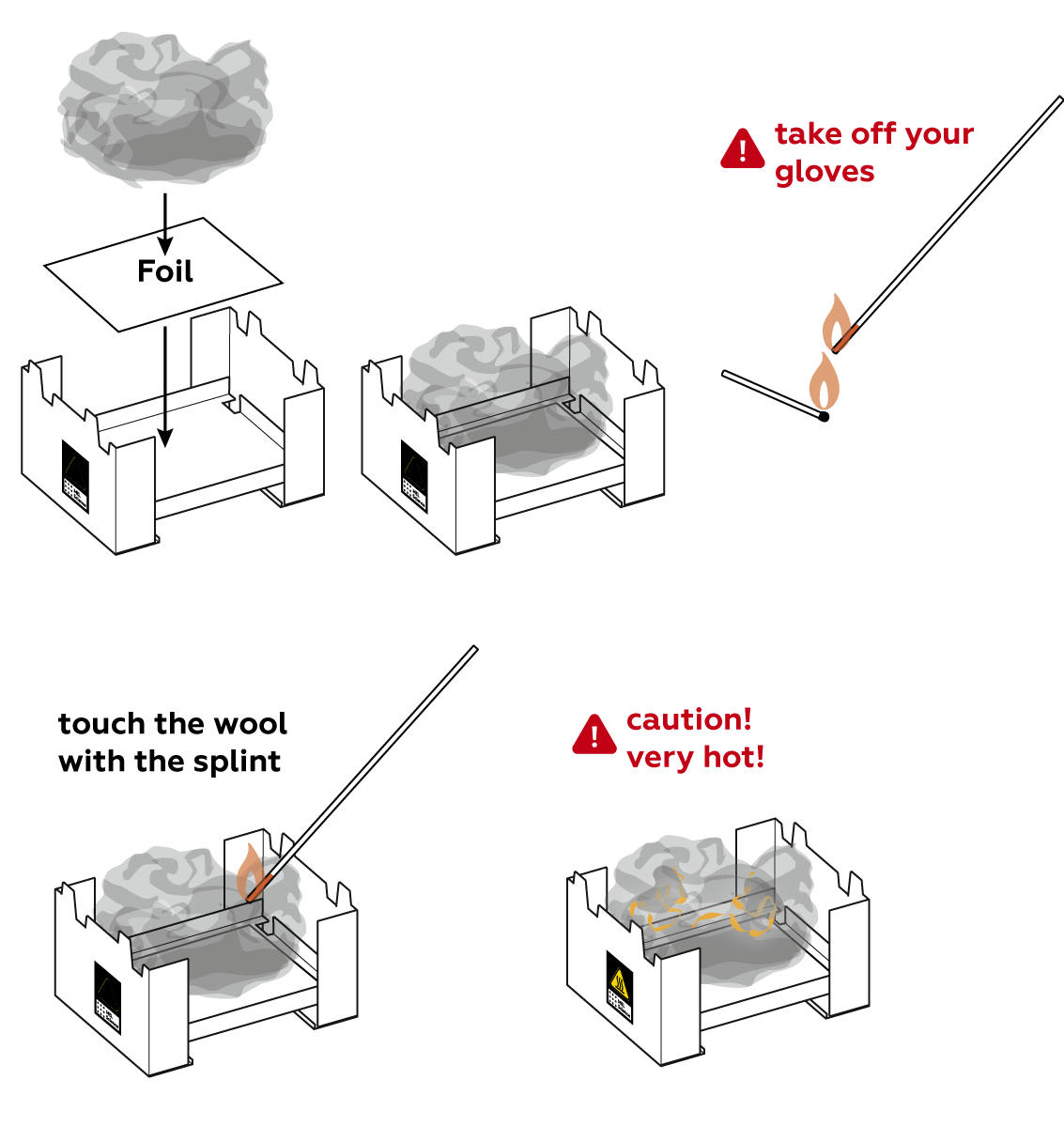
It's going to be quite hot, so put the iron wool in your stove. Ignite the iron wool.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage.
Scientific description

by the bulk of the metal. Fine threads of iron wool, however, do not have much bulk to them. Heat quickly accumulates in one place


, which eventually bind with oxygen O2

, making what's left of the paper lighter. Iron atoms Fe
, on the other hand, stay where they are and oxygen O
binds to them instead, making the wool heavier.
That’s interesting!
What’s the difference between iron and steel?
In everyday life, people often use the words "steel" and "iron" as synonyms. However, in practice, they are quite different.










