Pure hydrogen
Thymol blue is reduced with hydrogen


Safety
-
Put on protective gloves and eyewear.
-
Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Why does the foil float up to the surface?
A reaction of aluminum with sodium hydroxide yields hydrogen, which in turn is released in form of gas bubbles and pushed the light foil up. By the way, thanks to this property, hydrogen has long been used in aerostatics! Learn more about this phenomenon in “It is interesting” section.
The solution doesn’t turn colorless. What should I do?
Perhaps, you used a very dense foil for this experiment, and the reaction will require more time. Wait for 5–10 minutes more.
There is something dark left on the bottom of the test tube. What is it?
Since the quantity of sodium hydroxide wasn’t measured exactly, the foil might have not reacted completely. In order to fully dissolve it, add some more alkali.
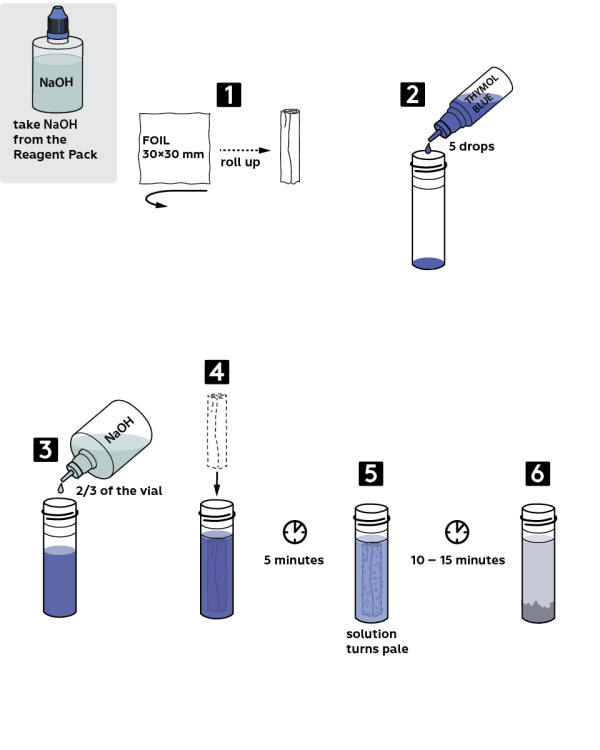
Step-by-step instructions
- Take a 30x30 mm piece of foil and roll it into a small tube.
- Add 5 drops of 0.001M thymol blue solution into a plastic vial.
- Fill the test tube 2/3 full with sodium hydroxide NaOH solution.
- Dip the foil roll into the vial. Wait 5 min, and the solution will turn pale.
- Wait 1015 min more.
- The foil will dissolve almost completely, and the solution will change its color to pale-grey.
Disposal
Dispose of the reagents together with household waste. Pour solutions down the sink and wash off with excess of water.
Scientific description
What happened to the foil?
Shiny aluminum (Al) foil reacted with diluted alkali (NaOH), which resulted in release of hydrogen (H2). Consequently, aluminum turned into sodium tetrahydroxyaluminate (Na[Al(OH)4]):
2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2↑
Unfortunately, the solution in our test tube is weakly alkaline, and sodium tetrahydroxyaluminate is unstable in such an environment. Eventually, most of sodium tetrahydroxyaluminate will decompose into sodium hydroxide (NaOH) and aluminum hydroxide (Al(OH)3). The latter is almost insoluble in water. Due to gravity, its particles slowly precipitate, thus, forming a cloudy layer at the bottom of the test tube.
Why did the solution turn colorless?
As mentioned above, a reaction occurred between aluminum (Al) and sodium hydroxide (NaOH) solution:
2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2↑
This reaction yields hydrogen. When just released in the reaction, a hydrogen atom is at its highest potential to donate one electron (and thus to become a reducer). And this is exactly what happens inside the test tube. As soon as formed, hydrogen reduces a thymol blue molecule turning it into a colorless substance. That is why we see the solution lose its color.
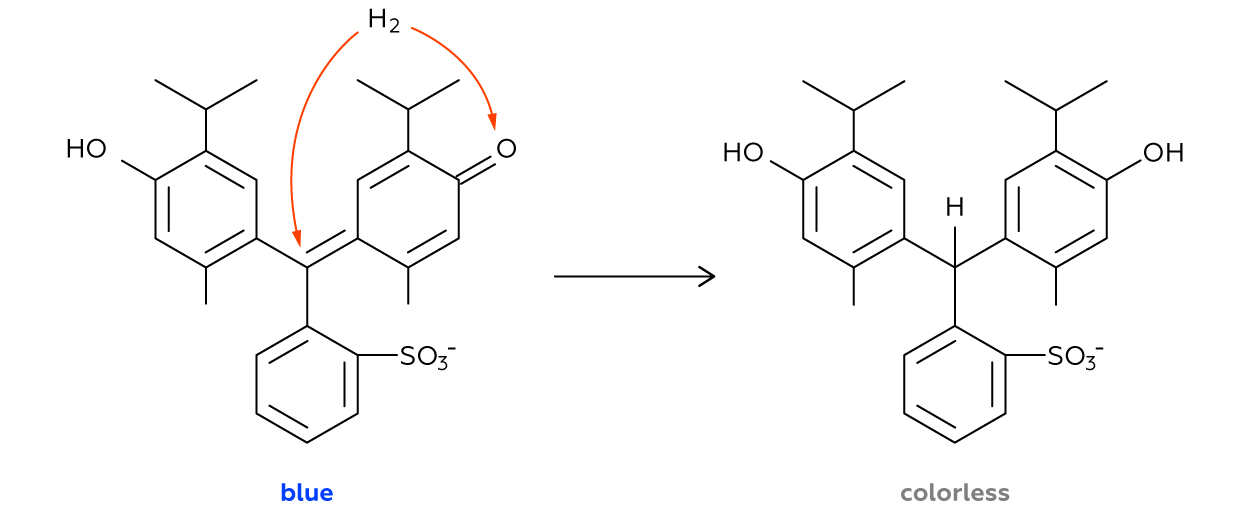
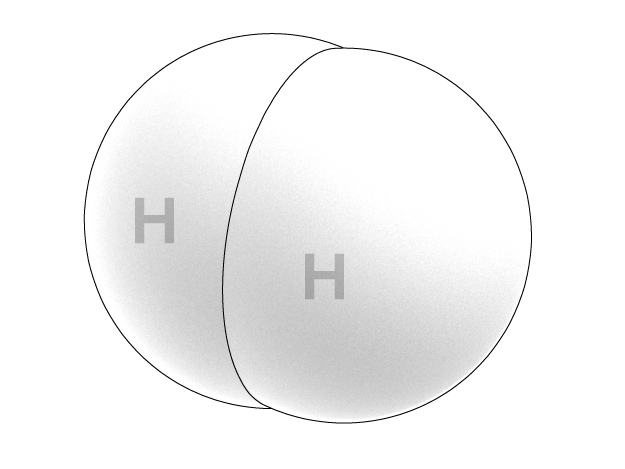
As we already know, a molecule of hydrogen consists of two identical atoms. However, not all of us are aware that at the moment of its generation, for the very first moments of its existence, a hydrogen atom is dwelling “in lonely pride.” And this atomic hydrogen can effectively reduce organic compounds! Obviously, it is disadvantageous for hydrogen to exist in form of isolated atoms because without a neighboring atom, it takes too much energy for a hydrogen atom to retain its only electron by the nucleus, all by itself.

As soon as two of such atoms form a joint hydrogen molecule, their reactivity drops dramatically. And the reason is that now two nuclei participate in retaining each of two electrons so that now they are connected much stronger. Therefore, molecular hydrogen cannot reduce thymol blue under the same conditions that work fine for atomic hydrogen.