Sulfur dioxide
Synthesize sulfur dioxide and watch as it makes the indicator change colors!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray and in a well-ventilated area.
- Keep a bowl of water nearby when working with fire.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
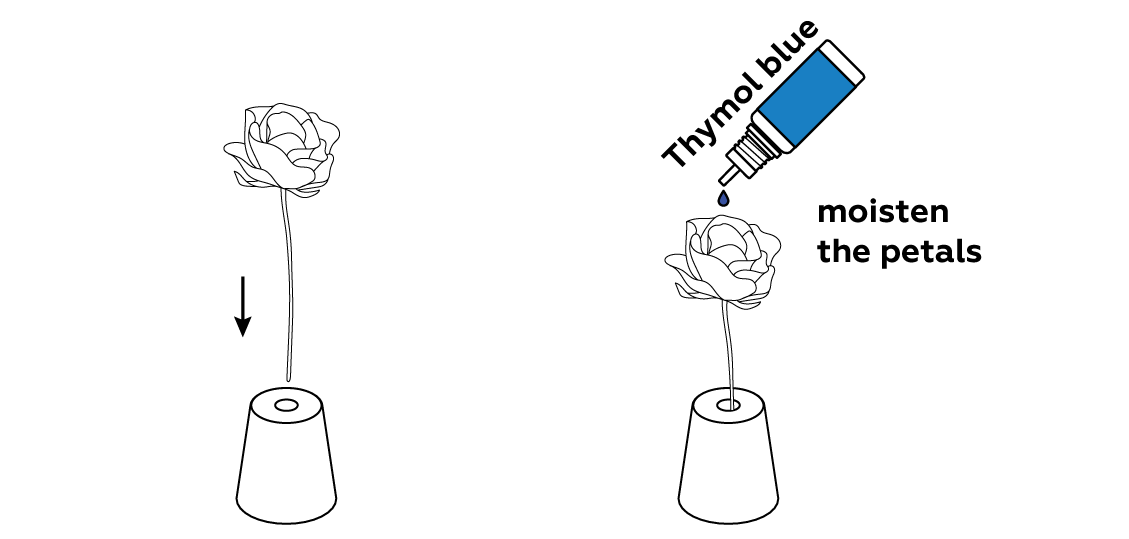
When the thymol blue is applied, the bud should turn bright blue. Be sure to apply enough indicator to cover the petals completely.
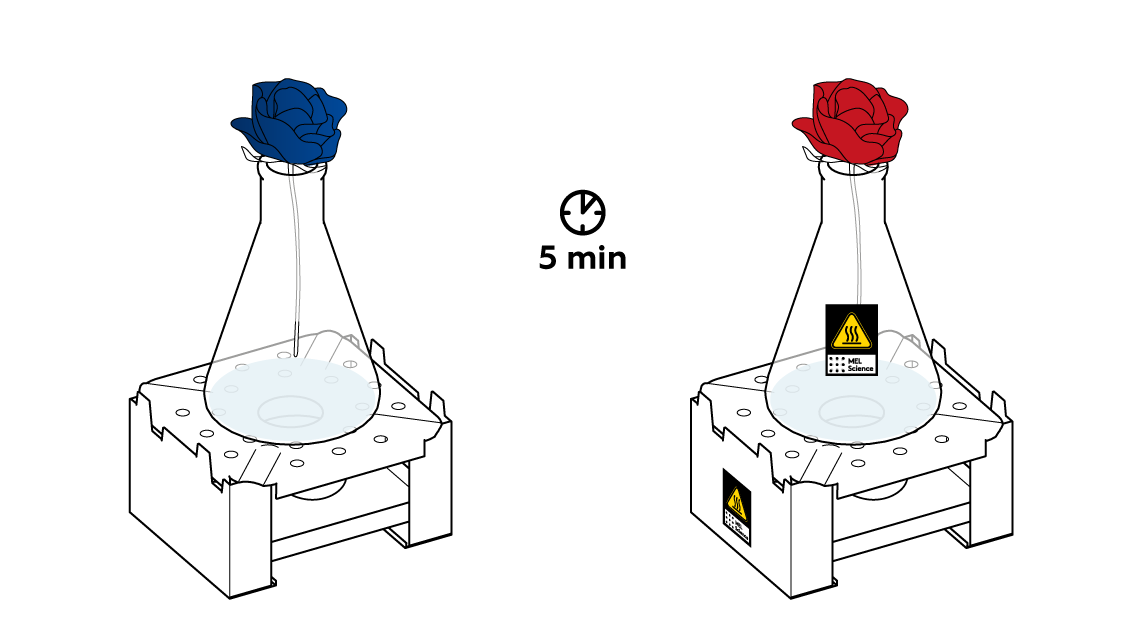
First, check that the candle is still lit. Next, make sure that the flower is positioned right above the neck of the flask. It’s important that gas flow right into the bud. Wait a little bit more.
Heating greatly increases the reaction rate. Without heat, the reaction will proceed very slowly.
Step-by-step instructions
Thymol blue is an acid-base indicator: it changes colors when it encounters acids or bases. It turns blue in a basic medium, and red in an acidic medium. As you can see, your flower is blue, meaning that the solution you soaked it in was basic.
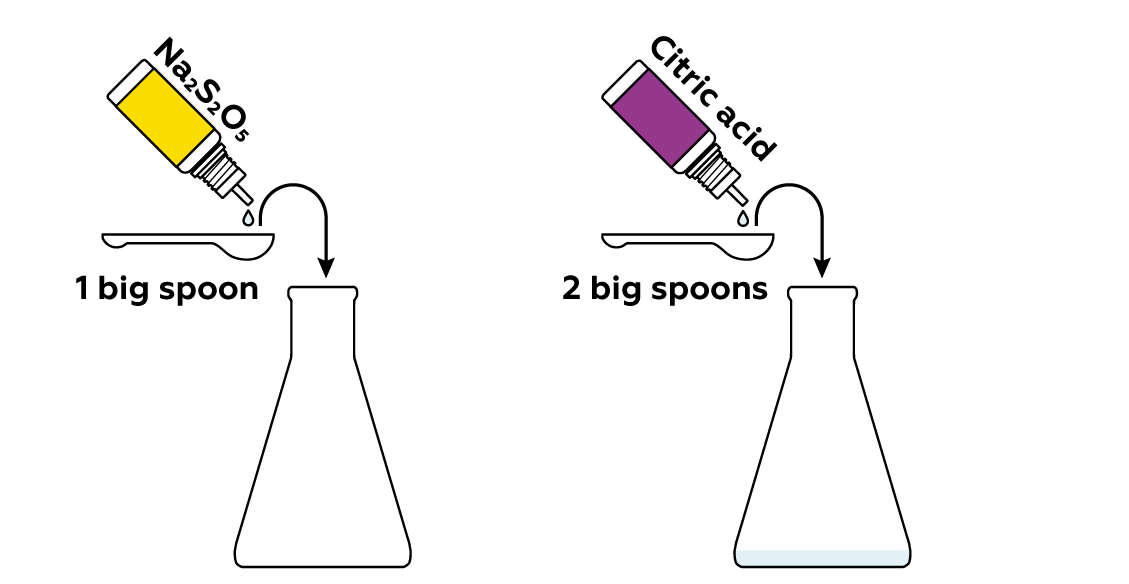
Sodium metabisulfite Na2S2O5 reacts with acids, such as citric acid, to produce sulfur dioxide SO2 gas.
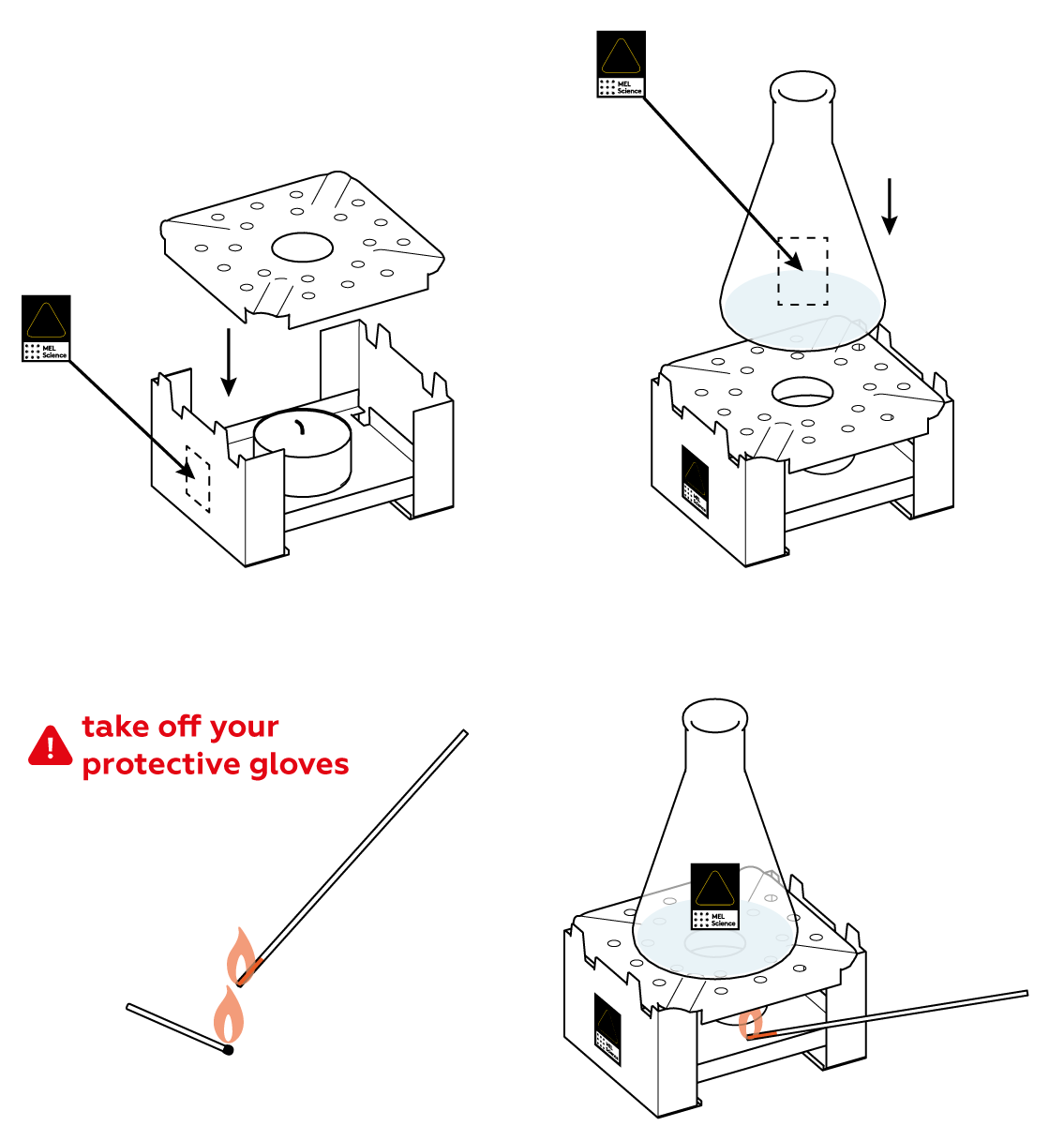
The reaction between Na2S2O5 and citric acid happens rather slowly at room temperature, barely releasing any SO2 gas. Heat the flask a little bit to speed things up.
Now, when the SO2 gas is emerging from the flask at a decent rate, put your blue flower into your "vase."
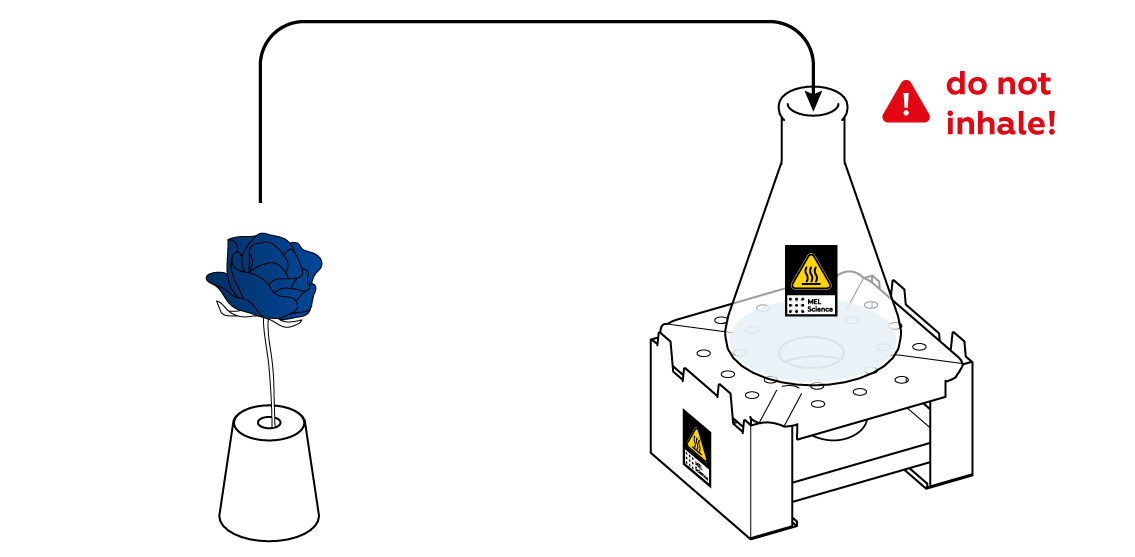
You didn't apply any acid to the flower, so why did it change colors?
Transfer your flower to the beaker and stop the reaction before you move on to the explanation.
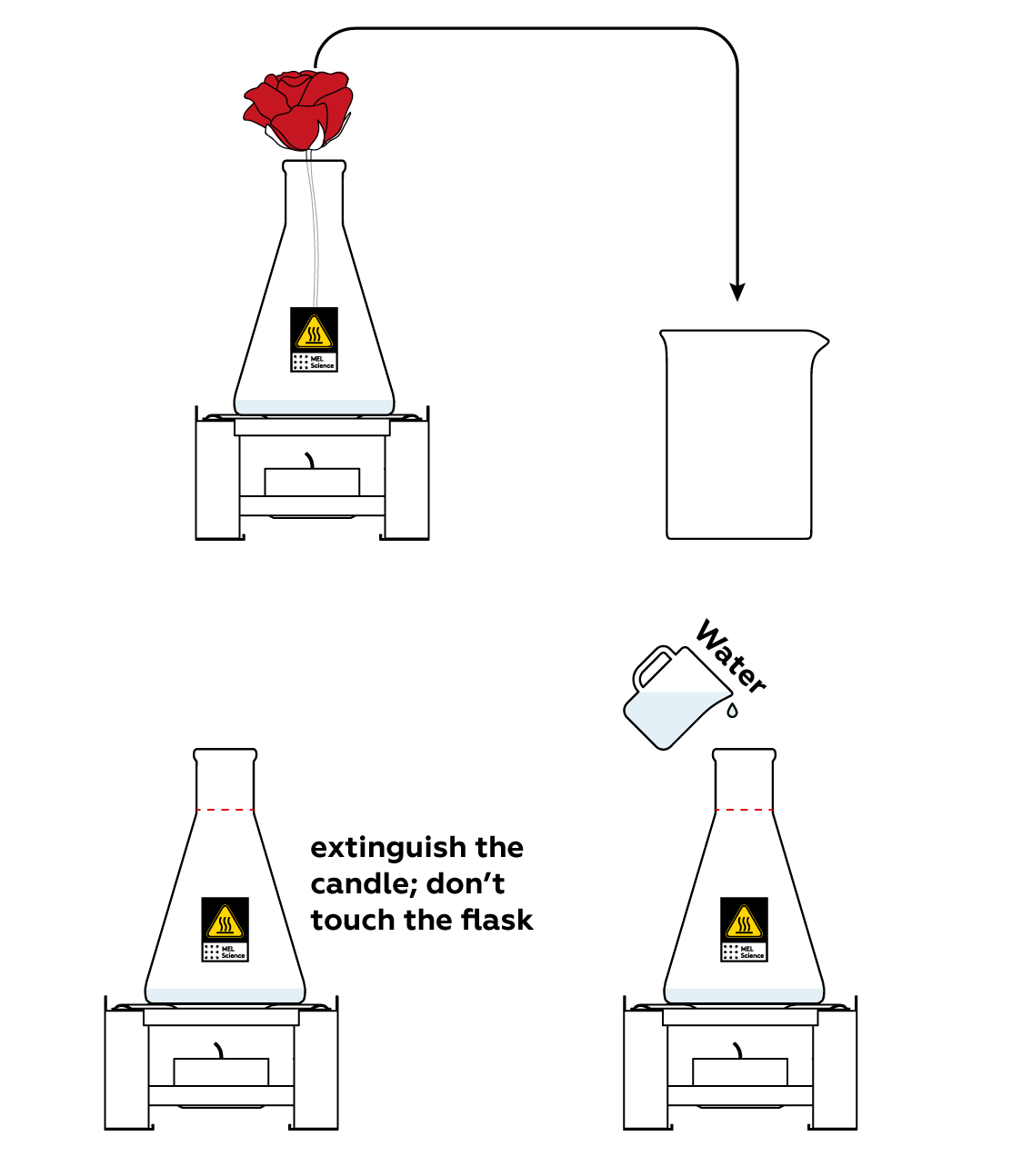
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description


—it turns into sulfurous acid H2SO3



into H2SO3



, making the flower less acidic. You can observe this as a change in the indicator's color from red to yellow

SO2 isn't the only sneaky acid-forming gas. The NO2
from the neighboring experiment does something similar: it binds with water H2O

and nitrous HNO2

also binds with water H2O
, but forms a base—ammonium hydroxide NH4OH
.
That’s interesting!
Sulfur dioxide is a colorless gas with a pungent, suffocating odor. It is also highly toxic – inhaling its vapors will invoke a cough, runny nose, and a sharp tickling sensation in the throat. In light of this, incorporating it in food may not seem like the best idea! However, it is widely used in the food industry as a preservative. Interestingly, it was first incorporated in food in the Middle Ages. The modern era has seen the advent of legal limits regarding the maximum content of this substance in food products. Limited quantities of this substance can be found almost everywhere, including in fruits and vegetables (canned, dried, and frozen), meat, drinks, some sweets, sausages, and products made of mushrooms and potatoes. Moreover, sulfur dioxide is considered an excellent stabilizer and antioxidant. It prevents the proliferation of bacteria in meat products and is also used to prepare fruits, both fresh and dried, for storage. Juices and other non-alcoholic beverages are almost always treated with sulfur dioxide to ward off mold and bacteria. Moreover, sulfur dioxide is quite commonly added to wine to stabilize its microflora, prevent unwanted fermentation and souring, and “fix” its color.