“Tin hedgehog” experiment
How to grow a metal hedgehog in 5 minutes
Many of you may have heard about animals out of a test tube, but what about a metallic hedgehog out of a beaker?
Safety precautions
Wear protective gloves, eyewear, and a mask. Perform this experiment in a well-ventilated area. Observe safety precautions when working with concentrated acids.
Reagents and equipment:
- piece of zinc;
- 5g tin(II) chloride;
- 70% acetic acid;
- distilled water;
- 2 beakers.
Step-by-step instructions
Combine 5 g of tin(II) chloride and 95 mL distilled water in the beaker. Watch as the solution turns cloudy. Add a few drops of acetic acid – the solution should become transparent.Tie the zinc pellet to a splint or pencil and immerse it in the solution. After 4 minutes, the zinc will be covered with spiky crystals.
Processes description
When tin(II) chloride is dissolved, its hydrolysis takes place, and an insoluble precipitate of tin(II) hydroxide forms. The 70% acetic acid is added to reverse the hydrolysis and obtain tin(II) ions.
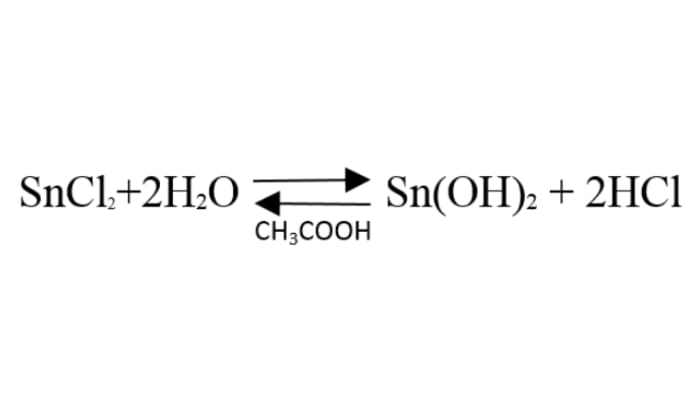
Zinc is to the left of tin in the reactivity series of metals, so it easily forces tin out of the solution. An oxidation-reduction reaction takes place, and the tin is reduced on the surface of the zinc in the form of beautiful spiky crystals.
SnCl₂ + Zn → Sn + ZnCl₂