Soap candle
Make a homemade candle!

Reagents
Safety
-
Put protective gloves on.
-
Conduct the experiment on the tray.
-
Take protective gloves off before lighting the candle.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
Step-by-step instructions
-
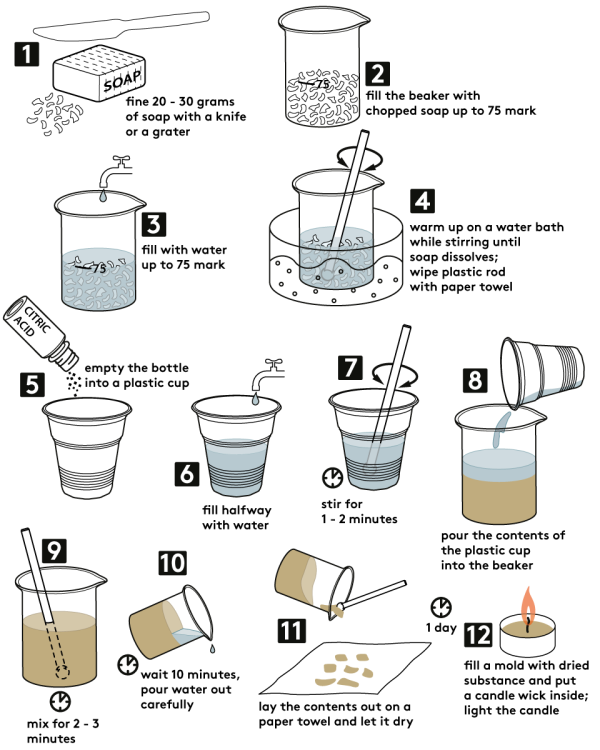
Take regular lye soap (a piece would weigh approximately 200 g). Toilet soap would also work for the experiment. However, the less additives and fragrance in the soap, the better. Scarify the soap marking it into 6—8 even sections. Fine around 30 grams of soap (one section) with a knife or grater.
-
Fill in the beaker with soap shavings up to "75" mark. If needed, grate some more soap.
-
Fill the beaker with water (preferably warm) up to "75" mark.
-
Further, dissolve the soap in water to obtain a thick homogeneous soapy mass. In order to dissolve soap, place the beaker with soap shavings in a preheated water bath. Stir the soap with a plastic stirring rod.
To make a water bath, use a bowl or a medium-sized pot. Put it onto a heat-resistant surface and place the beaker with soap shavings inside. Finally, fill the bowl or pot with boiling water, so that its level was slightly higher than the "75" mark on the beaker.
If the water bath cooled down, and the soap hasn't dissolved yet, take off the beaker (hold it only near the top!) and refill the bath with fresh boiling water.
You will need 30—40 minutes to dissolve the soap completely.
ATTENTION! Observe safety measures when working with boiling water.
When soap dissolves, dry the plastic stirring rod with a paper towel: you'll need it in the following steps. -
Take a plastic cup from the Starter kit. Pour the whole bottle of anhydrous citric acid C6H8O7 (10 g) into it.
-
Fill up the cup with water halfway.
-
Mix the contents with a plastic stirring rod for 1—2 min. until citric acid dissolves completely.
-
Pour out the cup contents into the beaker with dissolved soap mass.
-
Stir the resulting mixture for 2—3 min. with a plastic rod.
-
Leave it for 10 minutes. Then drain off the water carefully from the beaker. If the mixture hasn't yet separated into layers, leave it for 10—15 min. more, and then drain the water off.
-
Using a plastic stirring rod, lay out the contents from the beaker onto paper towels. Let it dry for 1 day.
-
Make sure that the mass has dried completely. First, grease the candle wick with a small amount of the mass obtained. Next, fill the candle dish with the mass and insert the wick as shown. Tamp the mass down in the dish.
ATTENTION! Take off protective gloves prior to lighting the candle.
Light the candle.
Expected result
You prepared a hand-made candle by educing stearic acid from soap.
Disposal
Dispose of the experiment residues along with regular household trash.
Scientific description
What is soap? What does it consist of?
Soap is a well-known solid or liquid detergent. What does it consist of and why does it help to clean one’s skin? It may sound weird but soap consists of quite complicated molecules. Each of these molecules carries a positively charged sodium ion Na+ (with which we are familiar) or a potassium ion К+ (in liquid soap), and a large negatively charged organic fragment (anion).
These anions resemble tadpoles, as they have a negatively charged “head” on the one end, and a long “tail” on the other—a chain of carbon atoms (approx. 15–20 atoms). When we dissolve soap in water, these “heads” readily put on a “watery coat”, just like Cl- ions would do.
At the same time, this long tail of a molecule does not feel comfortable in water and constantly tries to escape. It appears that most molecules of dirt behave the same way, and thus, they are hardly removable with just water. Otherwise happens when dirt gets in contact with soap solution, “like attracts like”: soap’s anions turn tails toward dirt particles, but their heads are left in water. Thanks to such mediation, it's much easier to wash out dirt with soapy water compared to just plain water.
Molecules similar to the one described above are called “salts”: you already know copper sulfate CuSO4 and ammonium chloride NH4Cl. In a solution, salts easily dissociate into positively and negatively ions:
NH4Cl → NH4+ + Cl-
CuSO4 → Cu2+ + SO42-
NaCl → Na+ + Cl-
Interestingly enough, regular cooking salt, or sodium chloride NaCl, the only compound we got used to call “salt”, does indeed belong to the class of salts. It's easy to get confused!
Finally, complex soap molecules are called fatty acid salts. More on them a bit later, however.
What is stearin?
In our case, stearin is the base of a candle. If we replace sodium ions Na+ in a compound from the previous topic by protons H+, we will obtain… stearin—a mixture of so-called fatty acids. Fatty acids feature a specific functional group consisting of carbon, oxygen, and hydrogen atoms on the one end, which makes them belong to acids group. Another end stays the same: a familiar chain of carbon atoms. Thus, by changing just one atom in a molecule, we can get two compounds with very different properties. One of them is able to wash out dirt, another could be used to make a candle!
Why do we need citric acid?
Since soap is salt of fatty acids, and candle consists of stearin (fatty acids themselves). Hence, to make a candle, we need another acid to react with soap solution. The reaction proceeds as follows: citric acid takes Na+ from fatty acid salts and in return provides H+, thus producing fatty acids, or stearin. Stearin is poorly soluble in water, so it forms a viscous sticky residue. After we dry out that stearin, we can make a candle.
Why is it important to dissolve soap before adding citric acid?
In order to turn citric acid into salt and fatty acid salts into fatty acids themselves, we need to create certain conditions, so that nothing would prevent them from meeting each other. If soap is not dissolved completely, significant amount of compound inside of big chunks would not get in contact with citric acid and wouldn't react. Stearin would be produced only on the surface of such chunks and it would isolate the core from the solution as stearin is insoluble in water. Therefore, it is very important to dissolve soap thoroughly in water prior to adding citric acid solution.
Thus, we need heating and agitation in order to accelerate dissolving soap in water.