Copper etching
Try printmaking on copper!


Safety
-
Put on protective gloves and eyewear.
-
Conduct the experiment on the tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Why do we have to secure the plates?
By securing the plates in playdough, we avoid any chances they would touch during the experiment. Thus, we eliminate the risk of a short circuit, which may be very dangerous. In case of a short circuit, the batteries may get overheated and even explode! On top of that, it would have ruined the experiment.
I didn’t get the drawing on the plate. What to do?
First of all, check the connections and their sequence in the circuit. The red crocodile clip should be connected to the red wire of the battery holder, and the black clip—to the black wire.
Then, examine whether the batteries are inserted in the holder correctly. If their polarity was set correctly, then try replacing the batteries with new ones.
Also, check the position of the crocodile clips: they shouldn’t touch the solution.
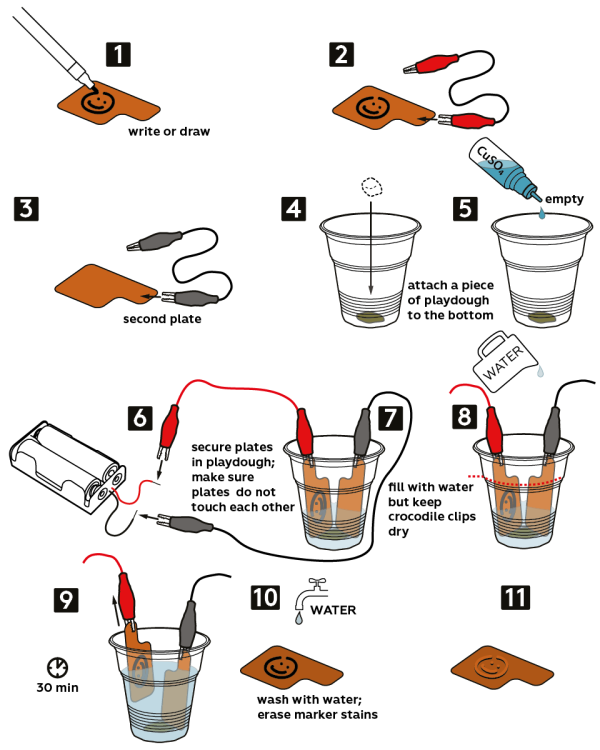
Step-by-step instructions
-
Take a marker pen and draw or write something on a copper plate, but avoid drawing on the “tab.”
-
Fix a red crocodile clip on the copper plate “tab.”
-
Take another copper plate and fix a black crocodile clip on its “tab.”
-
Take a piece of dark-colored playdough and knead it well. Now, attach this piece of playdough to the bottom of a plastic cup.
-
Pour all the 0.4M copper sulfate CuSO4 solution into the plastic cup.
-
Attach the loose ends of the crocodile clips to the battery holder: the black clip to the black wire, and the red clip—to the red wire. Insert 2 AAA batteries into the holder.
-
Fix the copper plates in playdough, so that they didn’t touch each other.
-
Add water into the cup to cover the drawing on the plates, but make sure the crocodile clips stay dry. Wait 30 min.
-
Remove the plates from the solution.
-
Wash the plate with drawing with water and erase the marker traces with a napkin or a paper towel.
-
The etched out drawing stays on the copper plate!
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with excess of water.
Scientific description
How exactly is etched relief formed on the copper plate?
What is relief? Relief (from the Latin relevo, meaning “to raise”) is a technique for creating a sculpture or an architectural element, which parts raise above the background plane of the same material.
To a certain degree, chemistry is different from other natural sciences in a way that work often starts from synthesis of new compounds and creation of new materials, and only after that their properties are investigated. Although in this experiment no new substances or materials are created, it allows us to balance on an edge between science and art by growing an etched relief via an electrochemical reaction.
So, in this experiment we have two conducting electricity elements – two copper plates – and copper sulfate solution that they have been immersed in. The solution itself also can conduct electricity, which by definition is a flow of charged particles. In the solution, copper sulfate completely dissociates into ions:
CuSO4 ↔ Cu2+ + SO42–
All the parts of our experimental apparatus can conduct electricity, which is a driving force for all the processes occurring in the experiment. The nature of these changes is relatively simple. With electrical current, copper atoms “jump” from the plate with the drawing to the clear plate. It happens by means of two electrochemical reactions:
Cu0 – 2e– → Cu2+
And
Cu2+ + 2e– → Cu0
With electrical current, the plate with the drawing gains an excess of positive charges. And that is why copper atoms can migrate from the plate surface into the solution, thus, turning into copper ions (the first reaction).
At the same time, with electrical current, the clear plate accumulates an excess of negative charges. Each copper ions approaching the clear plate from the solution withdraws a pair of electrons and settles on the plate in form of metallic copper (the second reaction).
Copper dissolves from the entire surface of the first plate, which is immersed in the copper sulfate solution. The only exception is the area with the drawing because of the marker coating that protects copper atoms from contacting the solution. And that is how the relief is formed. The plate “melts” everywhere except for the painted area. In other words, we created this etched relief element via electrochemical carving, by removing a thin layer of copper from the background plane of the resulting etched relief.
The possibility of carving different patterns on the copper surface made this method applicable for making jewelry. You can watch this process in this video.
Is it possible to perform copper etching without electricity?
Yes, it is! In this case, we need a strong chemical agent, which can easily dissolve both copper and copper oxide CuO, which forms on the surface of the copper plate. For example, ferric chloride FeCl3 solution can be used as such an agent, as shown in the video below.
In spite of the fact that we do not need any electrical batteries or other sources of electricity in this type of etching the chemistry behind it still includes electrochemical reactions:
Cu0 – 2e– → Cu2+ – this reaction is for the copper, which is being etched
Fe+3 + e– → Fe+2 – this reaction is for the etching agent
As you see, we have copper, which gives its electrons to iron turning into Cu2+ ions, while Fe3+ takes the electrons from copper turning into Fe2+ ions, maintaining the charge balance in the solution.
The results, which are obtained with the help of different types of etching, are pretty much the same, but there is one notable point to consider in the case of ferric chloride: it is very toxic, highly corrosive and acidic, this is why etching copper with electrochemistry is much safer.
That’s interesting!
How to cut sheet metal without scissors?
As it was mentioned in the main part, the etched relief was, in fact, cut out by removing excess copper layer from the plate. The same technique can be used to make a through hole in the copper plate!
To make a through hole, it is necessary to protect the plate with a scotch tape from all sides, leaving only one edge exposed for fixing a crocodile clip on. Then, make a small hole in the tape cover. After that, repeat the experiment according to the instructions, but this time let the reaction go longer – or five or more hours instead of 30 min. Then, copper will only be etched where the hole in the tape was made, thus, leaving a through hole in the plate.