Tartaric acid
Grow tartaric acid crystals

Reagents
Safety
-
Put protective gloves on.
-
Conduct the experiment on the tray.
-
Observe safety precautions when working with boiling water.
-
Take protective gloves off before lighting the candle.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
How to repeat the experiment?
Unfortunately, 20 g is the legal limit for the amount of tartaric acid allowed in a kit. To repeat the experiment, take the grown crystal out of the solution. Then, take the third vial of tartaric acid from the kit and add it to the remaining solution. Now you may run the experiment again.
Tartaric acid crystals don’t grow
Wait a little. Sometimes crystal growth starts later that we expect it to. Probably, there was a bit more water in the beaker than required – that could slow down the process.
Instead of growing on the wire, crystals form on the bottom of the beaker
In this case, take the wire out of the solution and let it dry. Don’t wipe it! This is important because doing so may leave scratches that may later act as crystallization centers. Now repeat the experiment: follow the instructions starting from the step 3.
Step-by-step instructions
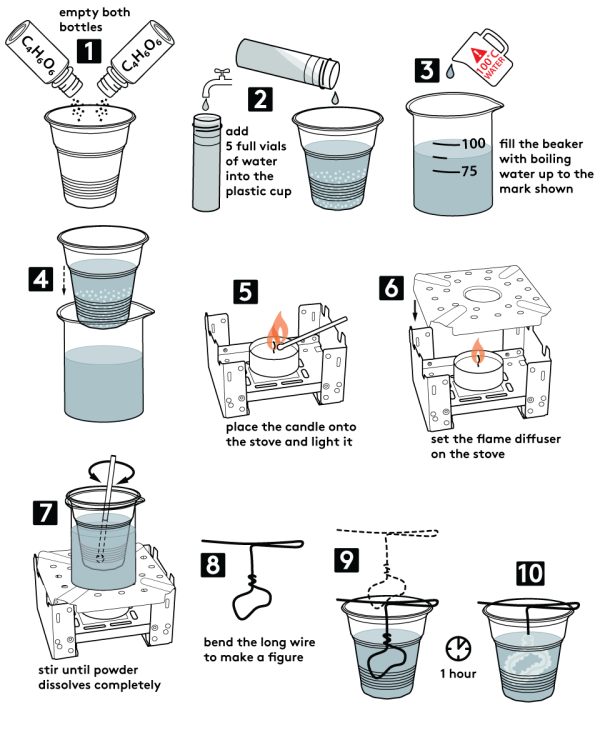
- Pour two bottles of tartaric acid into a plastic cup (20 g).
- Measure 5 plastic vials of water into the plastic cup.
- Fill the beaker with water up to the mark, as shown. Use caution when working with boiling water!
- Place the plastic cup inside the beaker.
- Remove gloves before working with open flame! Put a candle on the solid fuel stove and ignite it.
- Place the flame diffuser onto the stove.
- Place the beaker on the flame diffuser. Stir the plastic cup contents until all the tartaric acid dissolves.
- Form a long piece of copper wire into a figure. Make sure that it can be securely mounted inside the cup.
- Carefully remove the plastic cup from the beaker. Place the copper wire figure inside the plastic cup. Let the cup contents cool down at room temperature.
- Wait for about one hour. Tartaric acid crystals will grow on the copper wire!
Expected result
Tartaric acid crystallizes on a copper wire. Small crystals pile up, forming neat structures.
Disposal
Dispose of the experiment residues along with regular household trash.
Scientific description
Why do crystals grow?
Tartaric acid, as well as many other organic acids, is soluble in water. Though, it does not dissolve infinitely: at a certain concentration, a saturation point is reached. And then, no matter how hard we try, water cannot dissolve any more of it, even a single milligram, at a given temperature.
However, tartaric acid is one of those substances whose solubility increases upon heating. Therefore, when cooling a saturated solution, we create such conditions that concentration of tartaric acid becomes greater than “allowed” by its solubility. The “excess” of tartaric acid precipitates out from the solution. Since cooling process is gradual, tartaric acid doesn't just form a powder, but it has enough time to line up its molecules in a specific manner, forming bodies with a repeating pattern, or crystals. It is that systematic arrangement of molecules in crystals that makes them transparent and provides for a unique beautiful shape.
Why is it a wire where crystals grow on?
In this experiment, when a solution contains an excess of tartaric acid, its molecules have to choose from several options. The first is to remain in the solution. However, this state is unstable: it is too “crowded,” and the molecules “feel” uncomfortable when their concentration in the solution exceeds the solubility of tartaric acid at this temperature.
Consequently, tartaric acid molecules have to place themselves somewhere. “Looking” around, these molecules “see” water that they want to escape from, the tube surface, the wire, and their “twins” —the same molecules of tartaric acid. It appears that from the perspective of tartaric acid molecules, plastic, which the tube is made of, is uncomfortable and unpleasant to deposit onto, and the molecules are not attracted to it. Their neighbors, the same molecules of tartaric acid, are constantly moving, making it difficult to start developing a simple molecular ensemble right in the solution in the tube.
Among all the options, the wire seems to be the most attractive. It turns out that tartaric acid molecules can “stick” to the wire and feel quite comfortable on it. Thus, tartaric acid starts to stick to the wire. Subsequently approaching molecules place themselves adjacent to them, because the tartaric acid particles already deposited on the wire do not move, as opposed to those that remain in the solution. Since the “sticking” process occurs gradually, molecules are arranged relatively to each other in a correct order to form beautiful crystals.
Follow up
Crystals on a thread
Try to grow crystals of tartaric acid on a thread, rather than on the copper wire.
Repeat the experiment following the instructions. Instead of wire, in step 8, use a piece of thread, fastened on a splinter or match. Tie a couple knots at the end of the string and lower it into the tartaric acid solution. Now all you have to do is wait!
That’s interesting!
Optical isomerism: it all started from tartaric acid
Let's take a roundabout approach to the subject. Everything around us can be divided into two groups. The first group consists of objects that are symmetrical, so they look identical to their mirror image. Unfortunately, these objects are composing quite a minority group in our world. They often are simple geometric figures and shapes, such as a square, a circle, a sphere, a cube, or very, very small particles, such as atoms or molecules. These objects received an intricate name achiral.
The second group is much larger: it is represented by objects that differ from their mirror image. Strictly speaking, among them are all living beings, as well as most of nonliving matter. For example, a man or a cat does seem very symmetrical. However, upon closer examination, we can notice small differences in the shape of ears, eyes, hands or feet. Also, it is no secret that the inward parts of the body are not symmetrical as well: a heart and a stomach of most mammals (including humans, of course) are shifted to the left side, and a liver is located on the other (right) side of the body. Therefore, all human definitely belong to the second, or chiral, group, because they are different from their mirror image.
For very small particles, such as molecules, being a chiral or an achiral object is a critical characteristic. To better understand it, consider two simple molecules. A so-called tetrahedral arrangement is common for a molecule where one atom is bonded to four other atoms. It means that these four atoms are arranged around the central atom in a close packed manner. If we rearrange the molecule, so that the central atom and two (out of four) of its neighbors are in the same plane, then one of the remaining neighboring atoms would be above that plane, and the other under it.
Imagine that in the first molecule (on the left figure), to the central atom are attached two identical atoms (purple balls) and two other atoms, different from each other and from the first two (blue and red balls). Then, by rendering a mirror image, we obtain a molecule that is configured exactly the same as the original. Hence, the first molecule can be called an “achiral molecule”, because it is identical to its mirror image.
Now, consider the second molecule. It is similar to the first, but all the atoms around the central one are different (purple, green, red, and blue balls). If you make a mirror image of such a molecule, we will see a different molecule!
Finally, let's look at both molecules at a slightly different perspective: imagine the central atom in the plane of the drawing, two surrounding atoms above this plane (they are drawn larger on purpose), and the remaining two under this plane (they are drawn smaller).
Then we clearly see that the first molecule mirror image turns out to be exactly the same molecule. But the second (chiral) molecule mirror image gives a different structure, which cannot be aligned with the first, no matter how we rotate it.
It means that the right sides of both pictures show different molecules being on the different sides of an imaginary mirror. Substances consisting of such molecules will have different properties. Such molecules are called optical isomers, or enantiomers.
For the first time scientists faced the molecules chirality phenomenon through an example of tartaric acid. In 1834, it was observed that a solution of naturally occurring tartaric acid can refract light in a certain way. However, when tartaric acid had been artificially synthesized, it was found that such refraction does not occur anymore. An explanation was found in the crystallization of synthetic tartaric acid. There were two types of tartaric acid crystals, relating to each other just like an object and its mirror image. After these crystals were mechanically separated and dissolved in water, the light refraction effect was restored. However, for one type of crystals light was refracted to one side, and for the other type—in the opposite direction. This was the first demonstration of different properties of one compound, consisting of different chiral molecules.
Now, from the height of our knowledge, we understand why tartaric acid consists of chiral molecules. This is possible because the two central carbon atoms are precisely in such a position as the black atoms in our model molecules. For these carbon atoms, tetrahedral arrangement is typical, and they are bound with the atoms of different nature (oxygen and hydrogen) or in different positions (carbon atoms). As you can see, the mirror image of naturally occurring tartaric acid molecule is a different molecule, just like those that had been synthesized at the first artificial synthesis attempt. When they were mixed together in equal amounts in a solution, the refractive effects canceled each other out, and therefore, could not been observed.