Fox tail gas
Obtain nitrogen dioxide and collect it with activated charcoal!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray and in a well-ventilated area.
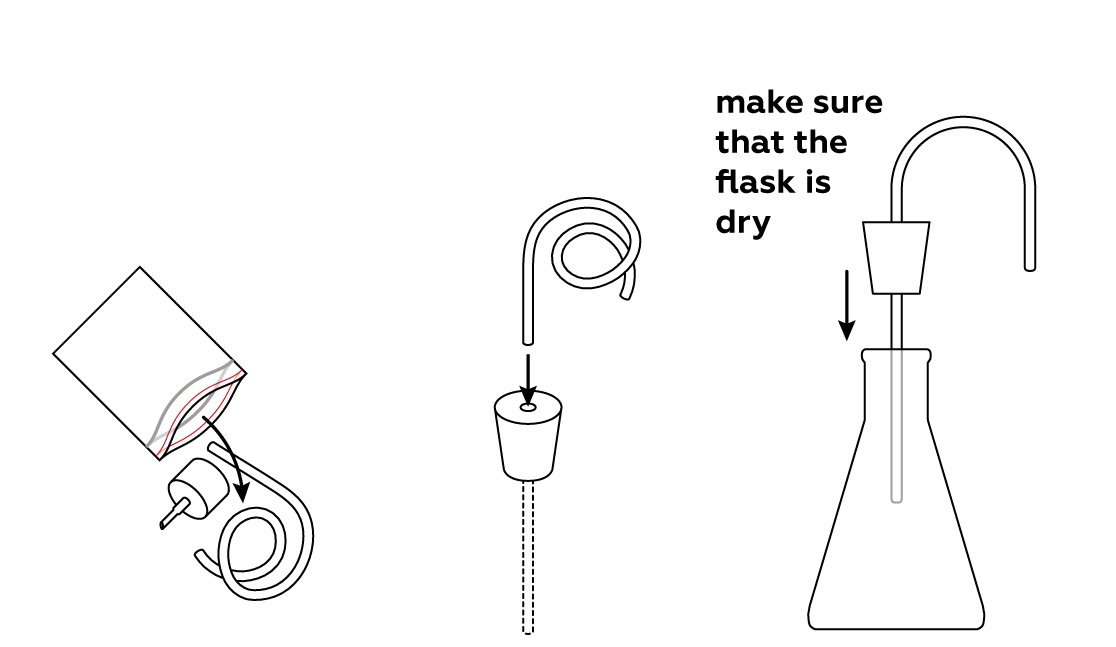
- Make sure your flask is clean and dry.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Don’t worry, it can be tricky. Keep in mind that you can always ask an adult for help!
Nitrogen dioxide NO2 is highly soluble in water, which means it will readily dissolve in water to yield nitric and nitrous acids. Thus, you may not see any gas if the flask is wet.
First of all, make sure you are using a dry flask. Nitrogen dioxide NO2 is very soluble in water, which means it will readily dissolve in water to yield nitric and nitrous acids. Hence, you may not see any gas if you use a wet flask.
If this isn’t the case, it’s possible your water has cooled down too much to be effective. Carefully pour it out and fill the beaker up to the 100 mL mark with fresh hot water. Wait 5–10 min.
Still isn’t working? Carefully open the vial and mix the contents again with a stirring rod. Close the vial and re-immerse it in the hot water.
Step-by-step instructions
This experiment will release some nitrogen dioxide NO2 gas, so be sure to perform it in a well-ventilated area.
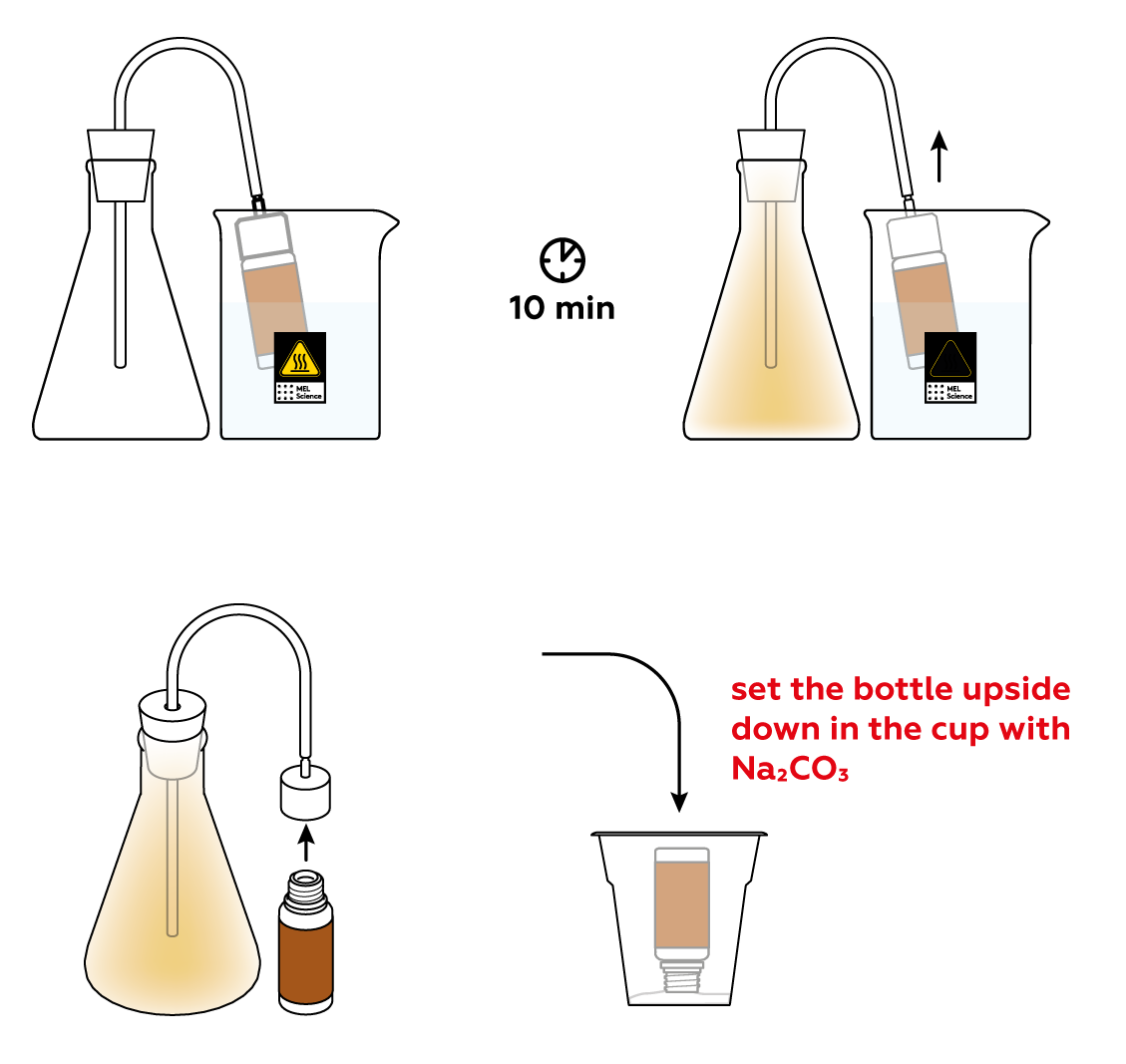
Assemble your gas-collecting apparatus.
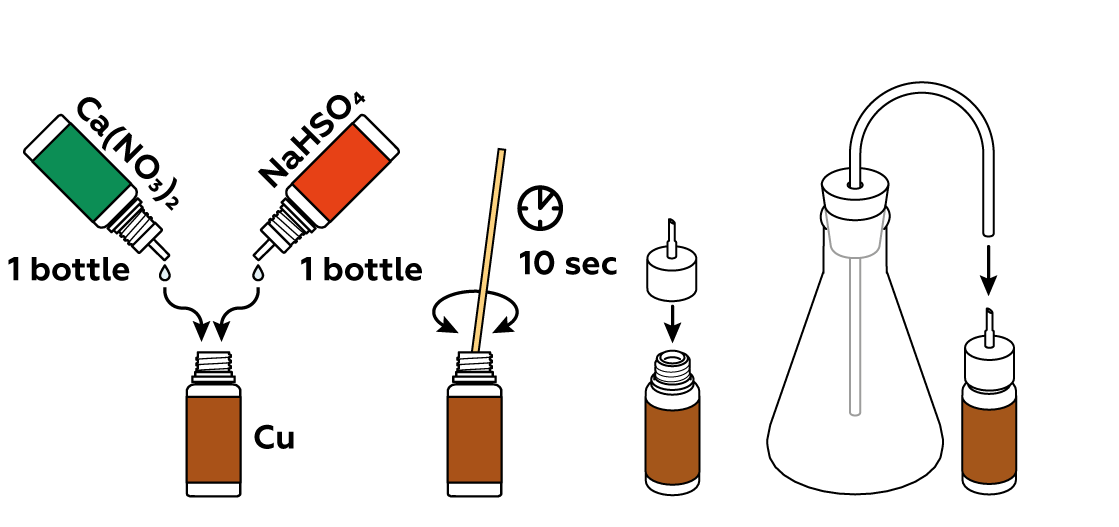
To obtain nitrogen dioxide NO2, you must first produce some nitric acid HNO3 by reacting Ca(NO3)2 with NaHSO4. HNO3 then reacts with copper Cu to produce NO2.
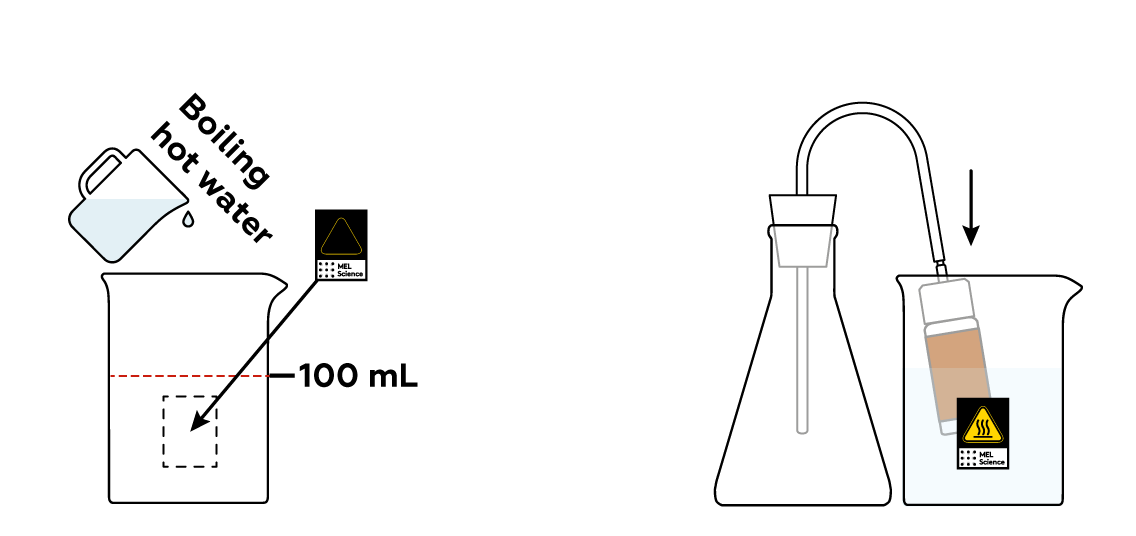
As is often the case, the reactions will proceed at a decent speed only at high temperatures. Let’s heat the bottle to get things going!
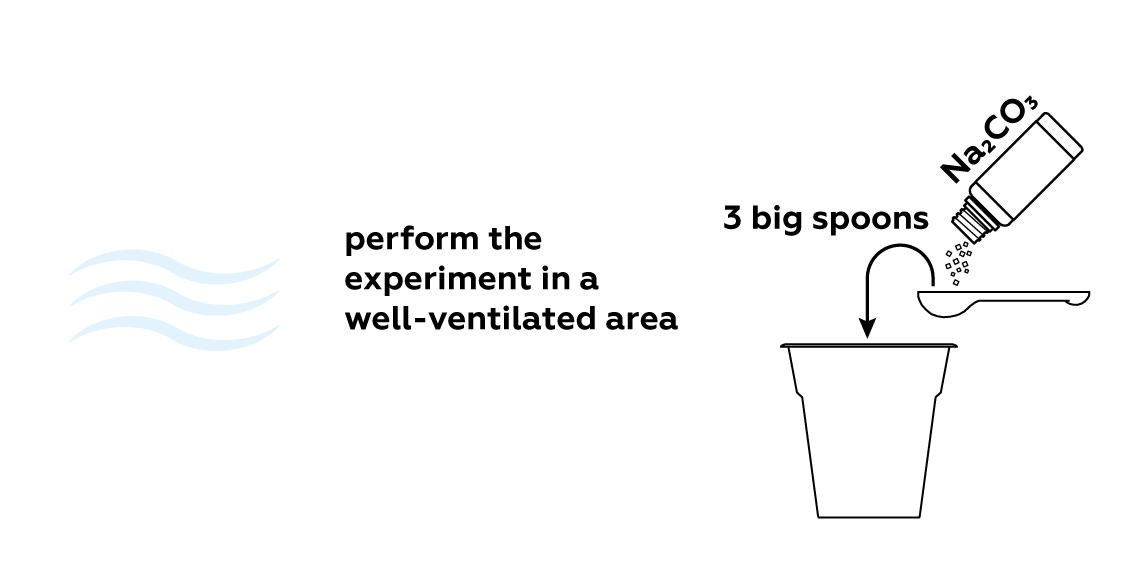
Unlike the gases that make up air, NO2 is vibrantly colored. Thus, it is relatively easy to tell how much gas is in the flask. Let the reaction run for about 10 minutes—this should be enough to obtain a decent amount of NO2 gas. Stop the reaction using sodium carbonate Na2CO3.
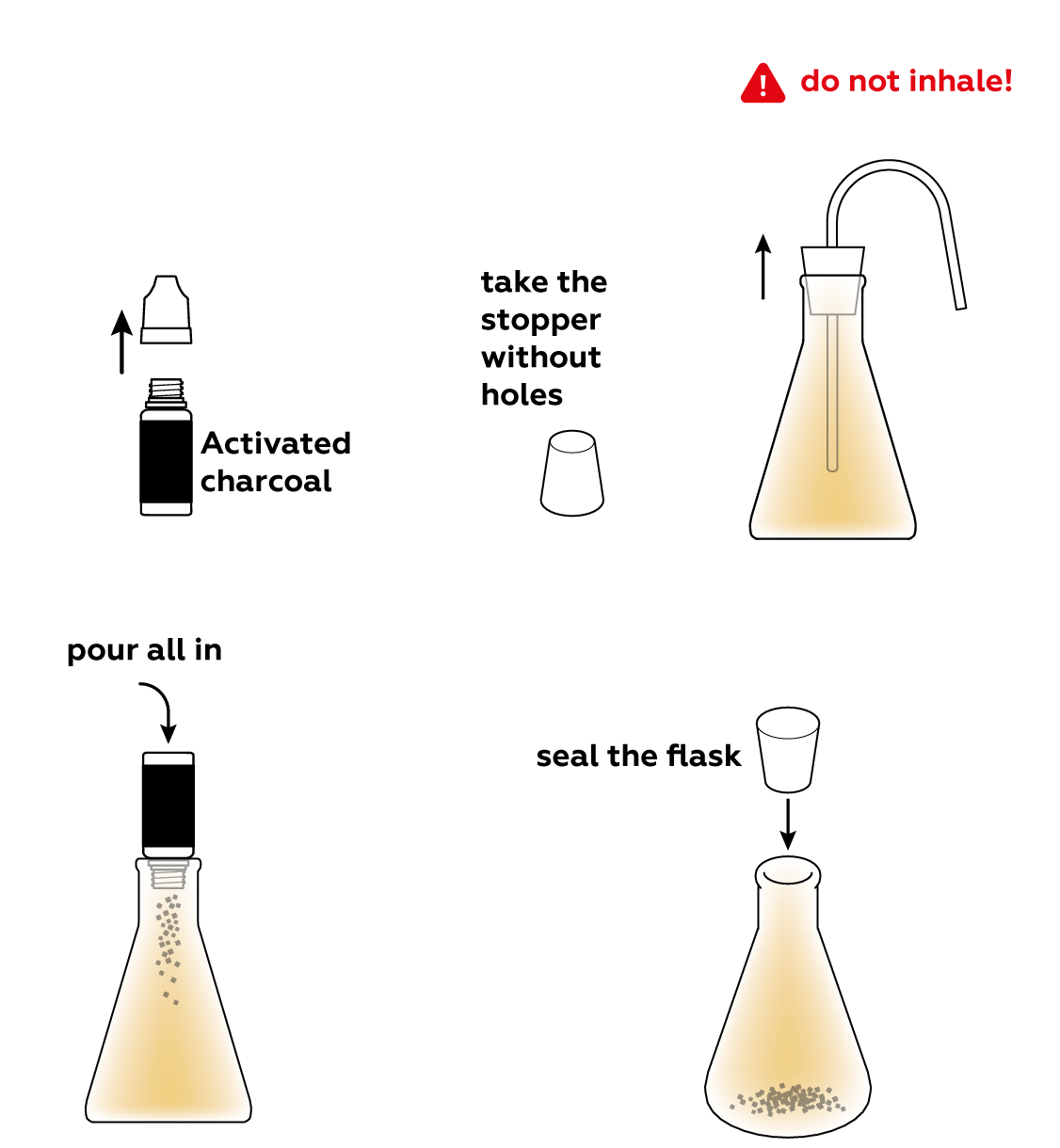
Activated carbon isn't very reactive and it won't react with the NO2 gas, but its superpower is not its reactivity. Add some to the flask and see what happens.
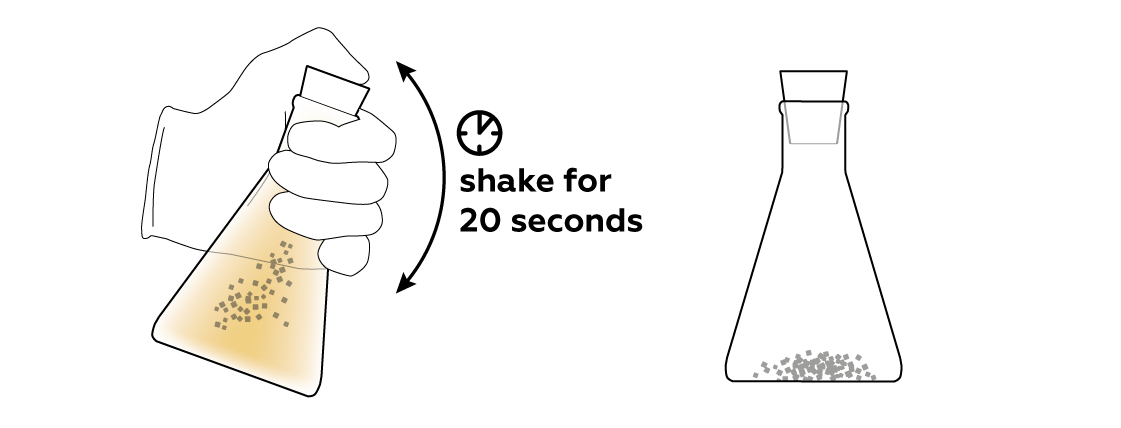
To help the activated carbon do its thing, shake the flask.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Gas molecules like NO2


To make enough room for all the molecules

NO2 is one of many potent air pollutants. Activated carbon, meanwhile, is used to clean industrial exhaust gases to make them less harmful to the environment.
That’s interesting!
What is adsorption?
You’re more than likely familiar with the phenomenon of adsorption. You encountered it the instant you got an inkblot on paper – or worse – on your clothes. You may have noticed that a painter’s clothes smell of paint, and a doctor’s gown smells of medicine. Adsorption is the process in which particles of one substance bind to the surface of another substance, and is utilized to purify drinking water and fix dye molecules on fabrics. Activated carbon is a powerful adsorbent used in gas masks, and is sometimes prescribed to treat food poisoning. Various adsorbents can be used to purify solutions when producing sugar, glucose, select medicines, and petroleum products. Moreover, they can be used to capture valuable vapors and gases. Even our perceptions of smell and taste depend on our nasal cavities’ and tongues’ ability to adsorb molecules!