Tea indicator
Learn how tea color is affected by pH!

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The more intense the tea color, the more noticeable the change. Green tea is quite pale, so the color transition won’t be very noticeable.
Step-by-step instructions
Make some black tea.
Divide the tea equally into three cups.
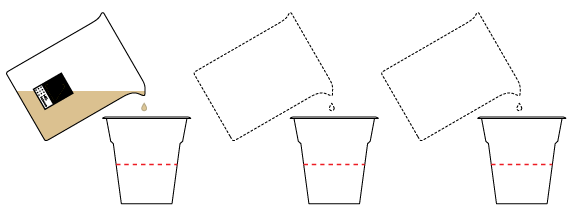
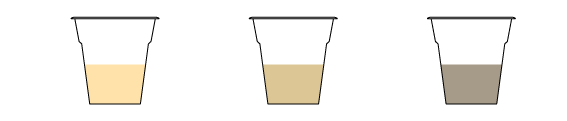
In the first cup, create an acidic medium by adding citric acid. Leave a neutral medium in the second cup. Create a basic medium in the third cup by adding sodium carbonate Na2CO3.

The color of the tea changes depending on its acidity, which means that the tea is a pH indicator.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Why does the tea lighten in an acidic medium and darken in a basic medium?
The leaves of black tea contain a large amount of thearubigin — the pigment responsible for the drink's color. Its structure has not been studied thoroughly, but it is known to change in an acidic medium, causing the tea to lighten. (You can observe the same phenomenon when you put a piece of lemon into your tea.) And when we add a basic solution like sodium hydrogen carbonate, the reverse happens: the tea darkens.
If we use green tea, the experiment results will not be so spectacular. The leaves of this tea are gathered before they produce enough pigment.
What is a pH indicator?
pH indicators help us determine the acidity, or pH value, of any given solution. A solution’s pH is determined by the concentration of hydrogen ions H+ and hydroxide ions OH- in it.
There exist many various pH indicators, which change their color depending on the solution acidity. Some of them change color only once. Take a litmus test, for example, which turns red at a pH of less than 4.5, and blue at a pH of greater than 8.3. Different shades of violet can be observed when the pH is between these two values, as the blue and red colors mix in different proportions. Other indicators can change colors several times, and the starker their transformations, the more accurate their indication of solution pH.
The pH of a solution can be determined with the help of indicator paper - special strips with a mixture of several pH indicators that change their colors according to the acidity of the solution. The strips are then compared to a special key, the values in which range from 0 to 14.
If a solution contains many free-floating protons, it will be very acidic (pH = 0). If there are almost no free protons, the solution will be basic (pH = 14). “Acidic” pH values (<7) will turn the indicating strips anywhere from red to a pale green color, while “basic” values will make them dark-green, blue or violet.
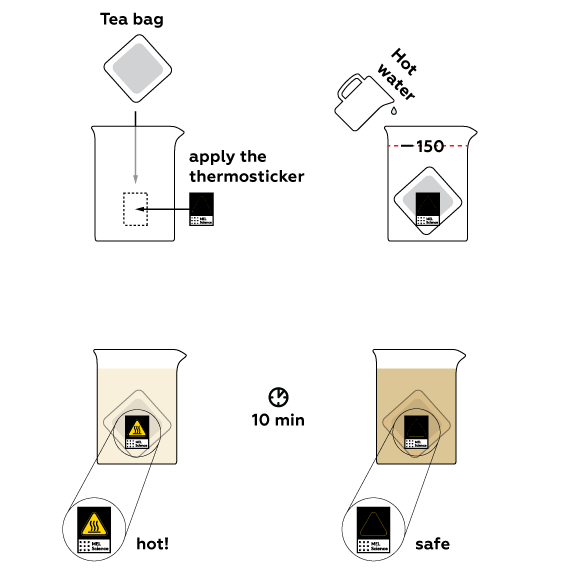
Why do we need the thermosticker?
When we added hot water, the thermosticker became yellow, and when the liquid cooled down, it darkened again. This sticker is a heat indicator; in other words, it can give us information about the temperature of the beaker and the substance in it. A cold beaker does not differ visually from a hot one, and one can easily get burned accidentally. Or even break the beaker, get injured, and/or spill the contents, though the beaker can contain substances even more dangerous than hot tea.
Sometimes, liquids can heat up on their own without our interference. For example, when we prepare an acid solution in water or dissolve inoffensive calcium chloride, the temperature of the liquid will increase without our help. This is because of the heat energy that accompanies the dissolution of a substance, which is released as a result of chemical processes.
That’s interesting!
Why are lemons sour
While peaches and strawberries can be considered desserts, some other fruits can make you grimace and pucker. Naturally, we’re talking about citruses. You already know that lemons are excellent at evoking those “sour” facial expressions. They taste even sourer than their relatives–oranges and grapefruits.
But where does its sour power come from?
The secret lies in their high concentration of citric acid (C₆H₈O₇), which is a weak organic acid found in many fruits and vegetables. In the scientific community, the intensity of citric acid and other substances is measured on the pH scale, ranging from 0 to 14. The pH value indicates how many hydrogen ions (H⁺) any given substance has. The smaller the number, the stronger the acid. Citric acid has a pH of 2, which is almost equal to the pH of the hydrochloric acid solution we know as “stomach acid” (1.5 - 3.5).
Oranges, grapefruits, and berries are all high in citric acid. However, of all the citrus fruits, lemons (and limes) have the highest concentration.
Watch this video to learn how to make a fizzy drink with citric acid: