Chemical reefs
Create a reef in your Petri dish!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
In the beginning, when using only 2 reagents, a small Petri dish will yield faster and clearer results. It’s better to use a bigger Petri dish when making new reefs with three or four different compounds.
To learn more about different compounds and their combinations, please read the scientific description of this experiment. But we recommend trying ammonium iron(III) sulfate (NH4Fe(SO4)2) and potassium hexacyanoferrate(II) (K4[Fe(CN)6]). Their blue reefs are quite striking.
It's great that you decided to try the experiment using other substances! Yes, this is possible for some pairs of compounds. Take, for example, CuSO4 and NH4Fe(SO4)2 or K4[Fe(CN)6] and Na2CO3. Combining these substances will only yield indistinct results.
This can happen if you reuse a Petri dish from a previous experiment without washing out leftover salt residues. This can make the results more ambiguous and less beautiful.
This is normal, actually. Stirring the salts will impede the experiment.
Try the experiment again, but this time just pour the compounds on the bottom of the Petri dish and leave them for a few minutes. The salts will dissolve in the water and form a reef.
Step-by-step instructions
This reaction will take place in an aqueous solution.

You will only need small amounts of the dry compounds.
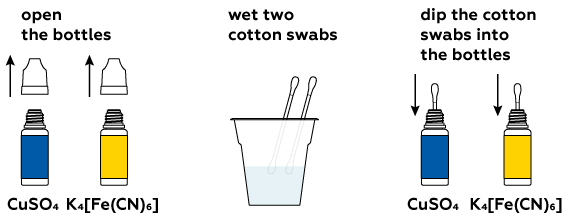
Let the crystals dissolve in the water.

Where the dissolved compounds meet, a reaction takes place and a nebulous brown “reef” appears.

You can use different combinations of CuSO4, K4[Fe(CN)6], Na2CO3 and NH4Fe(SO4)2 to create different kinds of reefs. You can even create four different kinds of reefs at once, but be sure to use a large Petri dish for that.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
When you see a chemical formula such as CO2, you can guess that one molecule of the compound 




Some combinations of ions, like CuSO4, break up easily in water (i. e. they dissolve easily), while other combinations are not so easily separated. When we put CuSO4 




Try combining different compounds and see which of them contain ions that can fit together to form "reefs". Blue Fe4[Fe(CN)6]3 
How can we combine other compounds?
Try combining different compounds and see which of them contain ions that can fit together to form "reefs.” Blue Fe4[Fe(CN)6]3 reefs are especially beautiful!
We recommend pairing the following compounds:
CuSO4 + K4[Fe(CN)6],
NH4Fe(SO4)2 + Na2CO3,
CuSO4 + Na2CO3,
and K4[Fe(CN)6] + NH4Fe(SO4)2.
What happens to these substances during the experiment?
First, the chosen substances dissolve in water. As they dissolve, they split into ions, just like the aforementioned copper sulfate. This is the case for each substance we deal with:
Copper sulfate: CuSO4 → Cu2+ + SO4–
Potassium hexacyanoferrate(II): K4[Fe(CN)6] → 4K+ + [Fe(CN)6]4–
Ammonium iron(III) sulfate: NH4Fe(SO4)2 → NH4+ + Fe3+ + 2SO42–
Sodium carbonate: Na2CO3 → 2Na+ + CO32–
We don’t aid them in this – but the ions really want to spread all throughout the solution. This phenomenon is known as diffusion.
Diffusion is a process wherein the particles of one substance (in this case, the ions obtained from the compounds we added to the water) spread out among the particles of another substance (in this case, the water molecules). In other words, the particles of two substances mix together. Through diffusion, these particles spread out to occupy all of the available surrounding space. Ultimately, the concentration (number per volume unit) of particles becomes uniform all throughout the system.
copper sulfate + potassium hexacyanoferrate(II) - a brown precipitate of copper hexacyanoferrate(II) Cu2[Fe(CN)6];
ammonium iron(III) sulfate + sodium carbonate - yellow precipitate;
copper sulfate + sodium carbonate - a light-blue precipitate of (CuOH)2CO3 and some other precipitates;
potassium hexacyanoferrate(II) + ammonium iron(III) sulfate - a vivid blue precipitate - Prussian blue Fe4[Fe(CN)6]3.
That’s interesting!
Two key processes in living organisms rely on diffusion – respiration, and nutrition. For example, as we breathe, oxygen and carbon dioxide molecules enter and exit blood cells via diffusion.
Unicellular organisms, and some types of cells in multicellular organisms, also use diffusion to obtain nutrition.
Let's learn a bit more about the role diffusion plays in breathing.
Amphibians, reptiles, birds, and mammals use lungs to breathe. Animals’ lungs vary widely in appearance, but their working principle is the same in both amphibians and humans – through the diffusion of gases into and out of their blood.
First, air enters the lungs and mixes with the air already inside. It comes into contact with the moist thin walls of the pulmonary vesicles, the alveoli, and permeates the blood vessels. In the blood, oxygen binds to the iron in red blood cells and is transferred with their help to all the cells of the body.
Cells of the body use oxygen for biochemical processes and convert it into carbon dioxide CO2, which is transferred back to the blood in the form of bicarbonate ions HCO3–. The blood then collects carbon dioxide from the cells and carries it to the lungs, where it is transferred back to the alveoli and exhaled out.
All gas transmissions from cell to cell are conducted via diffusion. This is also known as transmembrane transport because the process involves crossing cell membranes to get from one cell to the next. The diffusion of oxygen and carbon dioxide happens simultaneously, but in opposite directions – each gas will tend towards the side that harbors less of it.
Unfortunately, there are gases that bind to blood cells more strongly than the oxygen from the air. Take, for example, carbon monoxide CO, a toxic gas released during combustion. Carbon monoxide binds to the hemoglobin in blood better than oxygen and can stop oxygen transfer, preventing it from reaching blood cells and causing suffocation. This is what makes people suffocate in the midst of a fire or when a car engine is running in a closed room. Therefore, after people are evacuated from a burning building, they are given oxygen masks with oxygenated air to restore the oxygen content in their blood.

