Dyeing cloth
Let's dye some fabrics!

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Most probably, you haven’t washed the fabric thoroughly enough. Lather the samples and rinse with an excess of water. After such a wash, the only sample that should remain colored is the wool processed with eosin.
Polyester is whiter, it is slippery and shiny, unlike cotton. And wool is soft and a little itchy to touch.
It’s cotton. We supplied it for experiment follow-up.
Cut the strip into three even pieces. Take 3 disposable cups and put a piece of fabric into each of them. Now, add 15 drops of annatto solution into the first cup, then 15 drops of eosin solution into the second cup, and finally 15 drops of carmine solution into the third cup.
Further, add 5 drops of liquid soap into each cup. Fill the cups half-way with boiling water and wait 10 min. Then carefully remove the pieces of fabric, rinse them, and compare their color.
Step-by-step instructions
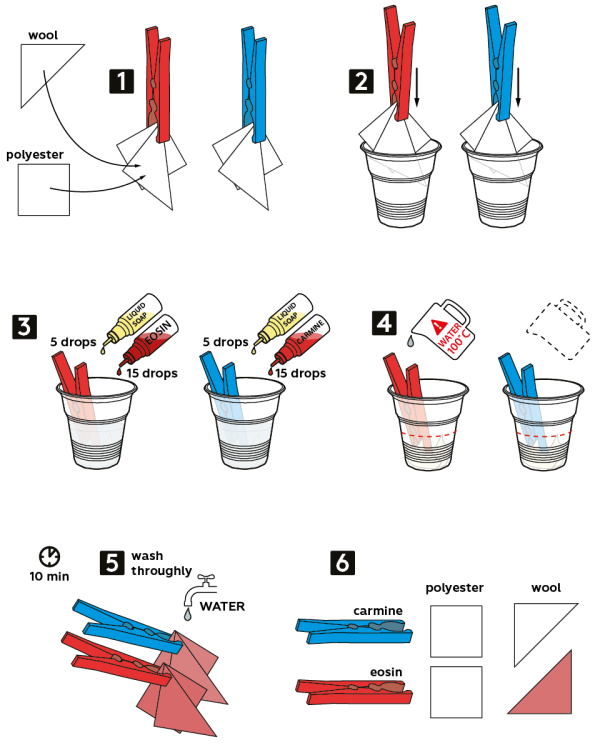
- Use the red clothes pin to clip 2 pieces of fabric—polyester, and wool. Do the same with the blue pin.
- Fix the samples in 2 disposable cups.
- Add 5 drops of liquid soap and 15 drops of 4% eosin solution into the cup with the red pin.
- Add 5 drops of liquid soap and 15 drops of 1% carmine solution into the cup with the blue pin.
- Fill the cups with boiling water to cover the pieces of fabric. Wait 10 min.
- Thoroughly wash the fabric samples with water.
- Compare results: eosin only colors wool fabric, and carmine easily washes off of both fabric samples.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
What is the difference between eosin and carmine?
The main difference between these two dyes is their chemical structure. This difference results in the way these dyes interact with different types of fabric.
The molecule eosin has a carboxylate group ─COO─ in its structure. Carboxylate groups can react with certain fragments in various kinds of cloth, for example, wool and linen. This reaction leads to a relatively strong chemical binding between the fabric and the coloring. So, the dye does not wash out easily!
Why are both of the dyes washed out of the piece of polyester?
Polyester is a type of fabric, which has no fragments that either carmine and eosin can bind to. This is why it is so easy to wash the both dyes out of the polyester.
Polyester is a fabric, which is made of very long molecules known as polymers. A polymer is like a very long chain made from many similar chain links, where each chain link is a chemical group. Many chains of polymers are then connected or crosslinked with one another to create a large surface area. Imagine a knight’s chainmail. In medieval times, a blacksmith used a similar pattern of crosslinks to make chainmail!
Polyester is a chemical term, which can be broken into poly, meaning many, and ester, a specific chemical group. Thus, the term polyester simply means “many esters”! Esters are chemical compounds, which result from the reaction between carboxylic acids and alcohol:
R1─COOH + R2─OH → R1─COOR2 + H2O.
Look at the following chemical schematic:
R1─COOH + R2─OH → R1─COOR2 + H2O
R1 and R2 = polymers (R simply means a long chain of chemical groups)
To make polyester fabric, the polymer fragment marked R1 has an extra carboxyl group -COOH and the polymer fragment marked R2 has an extra hydroxyl group -OH. These two groups react with one another to form an ester R1-COOR2. When hundreds of these groups react with one another, the result is the formation of hundreds of esters. This is commonly known as polyester (many esters).
Why does eosin dye the wool permanently while carmine simply washes out?
Wool is a natural fabric made from an animal product (as opposed to a synthetic manmade fiber such as polyester). Fabrics made from animal products are made of proteins. Proteins are simply long chains of chemical groups known as amino acids. Amino acids have chemical fragments, which link to one another across the protein chains to form a large surface area, in this case, wool. Eosin strongly binds to this link, while carmine does not. This is why, when the pieces of wool are washed, only the piece dyed with eosin remains colored.
Let’s take a closer look at what is happening at a chemical level. Wool is made out of proteins. Proteins consist of long chains of amino acids. Amino acids have different chemical fragments: carboxyl ─COOH and amino ─NH2. In a protein, a carboxyl group of one amino acid binds to an amino group of another, forming an ─NH─CO─ fragment. It is this ─NH─CO─ fragment that plays an important part in the coloring process. Eosin strongly binds to this fragment; carmine does not.
Follow up
One piece of fabric in the set is packed in a separate bag. Try coloring it with eosin solution, just like you did with wool and polyester. Have you succeeded? What can be concluded from the results of your experiment?
Ask adults to give you a piece of fabric or wool yarn. Repeat the experiment with using them with the same solutions (there is enough to color many pieces of fabric!).