Sugar in drinks
Is there any sugar in a soda drink?

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the tray.
- Keep a bowl of water nearby during the experiment.
- Place the stove on the cork hot pot stand. Do not touch the stove after the experiment - wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Indeed, this reagent tends to lump when stored. We have foreseen this case and supplied a wooden rod in the set, which you may use to crush the lumped salt.
Wait a couple more minutes - perhaps, dry substances haven’t yet melted completely. The qualitative reaction in this experiment requires high temperature. Compared to a solution, a melt has a higher boiling temperature. Thus, it is very important that you add only 1 drop of a drink, not a bit more. Unfortunately, if you’ve added more than that, then you would have to start over. Remove the beaker from heat, let it cool down, thoroughly wash it, then dry, and repeat the experiment.
Splendid idea! For the analysis, we only need one drop of a drink. However, you may try adding several different drops, just avoid mixing them. Use a clean pipette for each drink and keep track of your test drops. When you finish the test, tell us about your experiment. Upload a photo or video in social networks with the hashtag #melscience or simply email us at support@melscience.com.
Step-by-step instructions
To determine if any particular substance is present in a sample (like sugar, for example) we can perform a qualitative reaction. We can use concentrated sulfuric acid and urea for such a reaction. A substance similar to sulfuric acid H2SO4, sodium hydrosulfate NaHSO4, can be used instead of the acid.
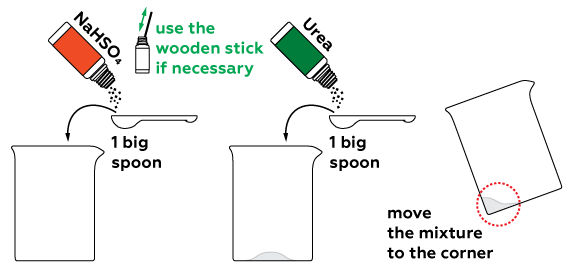
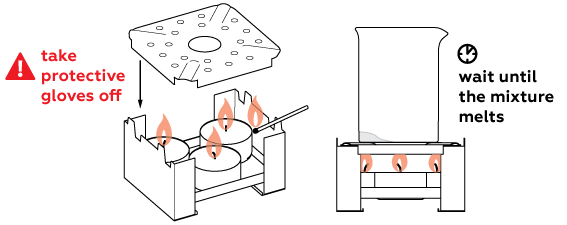
This reaction must be performed when the substance is melted, so the mixture must be heated intensively.
Now take a sample for analysis—some soda for example. One drop will suffice.
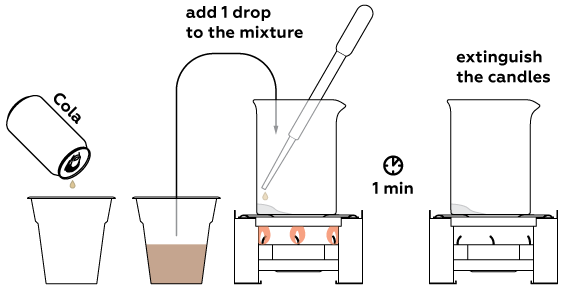
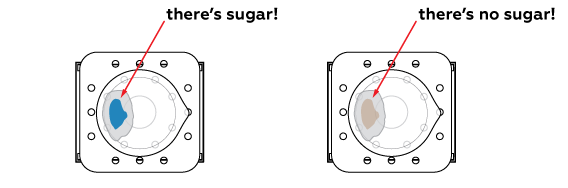
If the place where the drop fell turns blue, the drink contains real sugar. Otherwise, there is no sugar in the drink—it is sweet only because of artificial sweeteners.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
What reactions are classified as qualitative?
Chemists often study unknown powders or liquids or determine what admixtures are contained in a known substance. So-called “qualitative” reactions are often used for such purposes. These are short experiments that help determine the contents of the substances in question by analyzing changes in their appearance.
Qualitative reactions can vary widely, but their most important characteristic is the response (for example, the blue coloring of the melted mixture in this experiment), which we can observe as the result of this reaction.
This response helps determine which component is present in the composition of an unknown substance. This response is called analytical. The coloring of a burner flame while sample burning can be such a signal. When special reagents are added to a solution, one may observe a color change, precipitation, or the release of gas. Here are several interesting examples.
To determine if there are any potassium salts in a mixture, it is usually enough to put the sample into the colorless flame of a Bunsen burner. If potassium (ion K+) is present in the mixture, the flame will turn a characteristic violet color.
If a strong acid (for example, HCl - hydrochloric acid) is added to a solution containing carbonate ions CO32-, a colorless, odorless gas will be released - carbon dioxide CO2.
If a solution of sodium chloride NaCl is added to a liquid sample and white precipitate forms, it likely that the sample contains silver ions Ag+.
The effectiveness of qualitative reactions in finding any substances or particles often depends on the particles’ prevalence in a sample, the presence of various admixtures, and other factors. However, qualitative reactions help us study the composition of unknown substances quickly and easily.
For example, an iodine solution turns blue in the presence of starch. Such chemical reactions are useful not only in a lab but also in everyday life. For example, you can use this reaction to test the quality of cottage cheese. Natural cottage cheese does not contain starch; however, some shady manufacturers add starch to increase the mass of the product. If a little iodine is added to such “tainted” cottage cheese, the sample will turn blue.
The reaction of melted urea with a sample of a sweet drink is also qualitative: it helps us determine if there is sugar in the drink (or fructose, as you will see further).
What is sugar?
Sugar is a household name for the substance sucrose. This is a hydrocarbon, which consists of fragments of glucose and fructose connected by an oxygen bridge –О–. Sugar dissolves easily in water. In your body, it is deconstructed by the enzyme sucrase as soon as it enters your mouth.
What happens in the beaker?
At first, sodium hydrogen sulfate and urea melt. Approximately at 133o C urea CON2H4 melts and turns into a liquid. At the same time, various chemical transformations take place in the beaker.
When urea melts, its molecules almost immediately couple in pairs, forming a biuret C2O2N3H3:
2CON2H4 → C2O2N3H3 + NH3↑
Parallel to this, cyanuric acid:
3CON2H4 → C3O3N3H3 + 3NH3↑
Sodium hydrogen sulfate speeds up these reactions.
Why does the mixture turn blue when a sweet drink is added?
The sugar present in drinks interacts with the melted urea in the acidic medium, and the characteristic blue coloring appears.
This reaction is qualitative on one of the fragments of sucrose - fructose. This is why it can be used to test other substances for the presence of fructose. For example, lots of fructose can be found in honey and fruit juices.