Fats
Unsaturated fatty acids react with iodine and make it lose its color

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
There isn’t enough melted butter to fill half the vial.
Pour the melted butter back into a plastic cup and add another little piece of butter to it. Insert the plastic cup into the beaker and wait 3—5 min. Now, continue the experiment starting from step 5.
What vegetable oil would work best for this experiment?
Any liquid vegetable oil in your kitchen would be fine for this experiment. For instance, you may use corn, olive or sunflower oil.
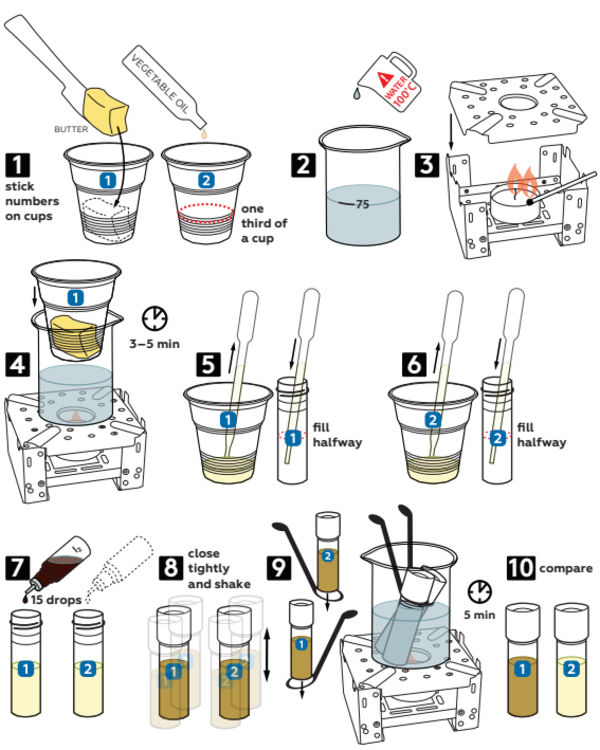
Step-by-step instructions
- Label two plastic cups with numbering stickers from the set. Fill each of cups 1/3 full with butter and vegetable oil, respectively.
- Pour boiling water into a beaker up to 75 mL mark.
- Place a candle into the stove and set a flame diffuser on top. Light the candle.
- Insert the plastic cup #1 into the beaker. Set the latter onto the flame diffuser and wait 3–5 minutes to melt the butter.
- Using a pipette, fill an empty vial halfway with melted butter.
- Take a new pipette and fill another vial halfway with oil.
- Add 15 drops of 0.05М iodine I2 solution into each vial.
- Tightly close both vials and shake them.
- Secure both vials in test tube holders and then put them into the beaker, as shown. Wait 5 min.
- Compare the color of resulting emulsions in both vials.
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
What is the difference between butter and vegetable oil?
Any organic oils (not only butter and vegetable oil) belong to one class of chemical compounds – fats. They may be different in color or form, and there is no surprise in this: the differences between vegetable and animal fats begin on the molecular level. A molecule of any fat contains a long chain of carbon atoms. In an animal fat, all of the carbon atoms are bound together by single bonds, while there are double bonds in vegetable oil chains. This is why vegetable oil is liquid and runny at room temperature, while butter, though seeming to be soft, does not lose its shape.
Is it possible to turn a vegetable fat into an animal one? Or, at least, make them more similar to each other? Of course, it is! For this, vegetable oil is “hydrogenated”: an atom of hydrogen is added to each atom of carbon, bound by double bonds. Because of such a process, all double bonds become single, as they are in the animal fat.
Why do we heat the cup with the butter?
Heating is needed to make the butter melt in order to take some for the experiment in a more comfortable way.
What happened when iodide was added?
The mixture with vegetable oil becomes colorless, while the one with butter remains the same color. Such a difference is also due to the different molecular structure of the fats. The molecules of vegetable oil contain “carbon-carbon” double bonds. This means that there are some carbon atoms, to which something else like iodine can be added. In the molecules of butter there are no such bonds, that is why iodine has nothing to bind with, and the color remains the same.