Atom size
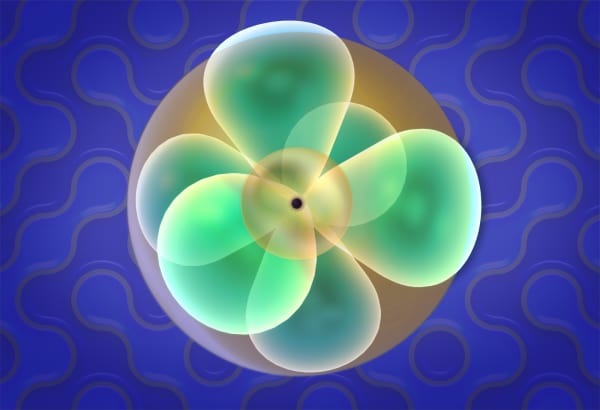
Students will see that electron orbitals look more like a fuzzy cloud of electron distribution without clear boundaries. So how is atom size defined? In this lesson, students will be introduced to several ways to define atom size.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

You know, that an atom is small, very small. How small is it?

Look at a sheet of paper. The thickness of it is one million atoms. What is one million?

Let's take one million sugar cubes and stack them on top of each other.

We get a tower above 30 000 feet, that is higher than commercial airlines fly.

Compare: one million sugar cubes and one million atoms.
So atoms are very small.

Let`s look at the hydrogen atom. Here it is.
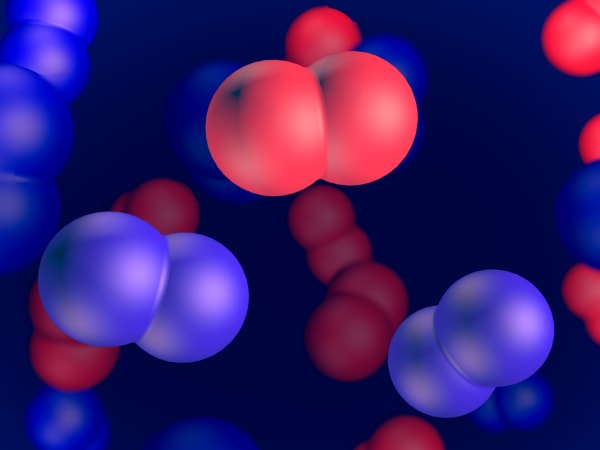
Before, we showed you atoms as balls.

But in fact, atoms looks like a spherical cloud. Like this.
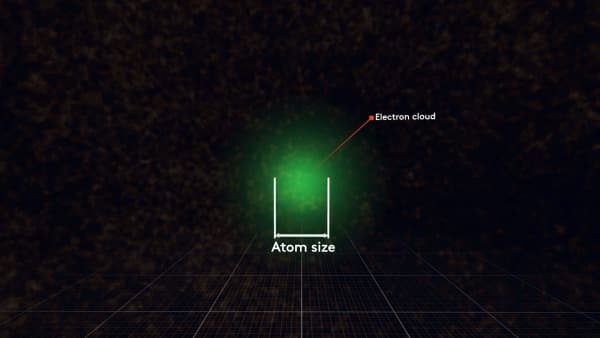
So how can we measure the size of an atom? Let`s try.
Maybe this is the size of an atom?

Or that?
It is a tricky question.
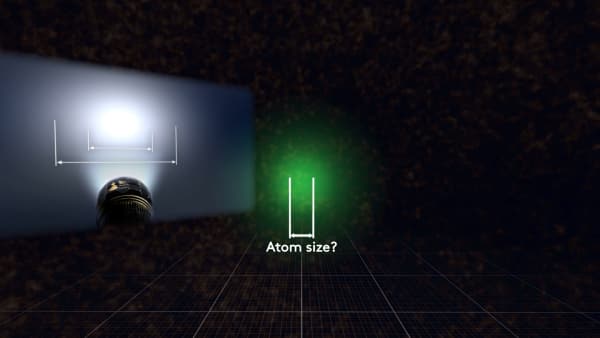
It`s like trying to measure the size of a light spot. What is the size of it?
This? Or that?
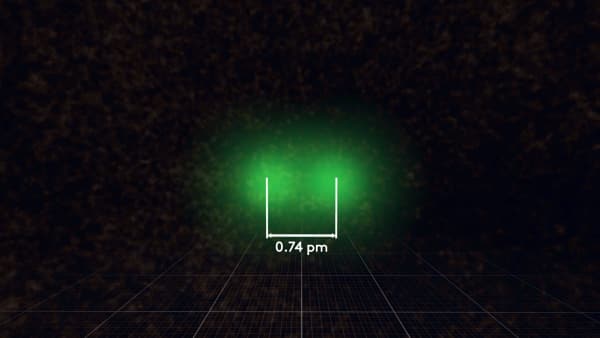
Scientists agree on how to determine the radius of an atom. We need two similar atoms combined in one molecule. For a hydrogen atom, it would be H2. Here it is.
We measure the distance between the nuclei of two atoms.

Then, divide it by 2.
Finally, we would call this - radius of hydrogen atom.

Different atoms have different atomic radius. The smallest atom is helium. And one of the largest atoms is cesium.

Now we go back to the lab. Some atoms exist separately and do not form compounds. They are called noble gases. For example, helium.
Helium in a balloon is a gas, consisting of individual atoms. For such atoms, the size can only be calculated theoretically.
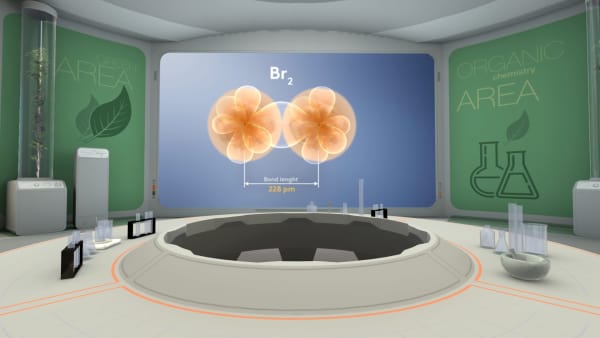
Bromine two is a real molecule. It consists of two bromine atoms. The distance between the nuclei of bromine atoms is 228 picometers.

Calculate the atomic radius of bromine atom.
Yes, you are right.
Teacher's notes
Keywords
atoms, atomic structure, electrons, atom size, atomic radius
Common misconceptions
- Atoms have clearly-defined size and spherical shape.
Students will
- Recall how small atoms are
- See the problem of measuring atomic size
- Examine the definition of atomic radius
Hands-on activities
Before VR
The aim is to give students a real-life example depicting objects that are hard to measure because you first need to define what their size would be.
This example asks students to measure the size of a light spot from a flashlight. Put a flashlight in a stand, so that the light spot shows on the table surface. Switch off the overhead light and ask the students to measure the light spot. Compare the results from different students.
History and sources of knowledge
- Experiments to measure atom size: Rayleigh experiment with oil.
- Modern methods: X-ray and spectroscopy.
Topics to discuss
- Not everything can be precisely measured.
- Importance of a definition.
- Different ways to define atomic size.
- Atomic size of metals.
Fun facts and quotes
- The atom with the smallest radius is helium. Helium was discovered not on Earth, but on the Sun. The atom with the largest radius is caesium, nearly 10 times bigger than helium.
Questions
- Give an example of something (not an atom or a light spot) where size is not obvious. How can we define its size?
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.