Plastics and iodine
Use iodine to differentiate between plastics!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay and in a well-ventilated area.
- Avoid inhaling iodine vapors from the beaker.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
This does happen sometimes. Use a wooden stick to loosen the reagent in the bottle.
Sodium thiosulfate neutralizes iodine, making the solution in the beaker safe to rinse down the drain. Visually, it makes the solution colorless and prevents it from staining the sink.
First, apply a thermosticker to the beaker – it will help you tell whether the water in the beaker is still hot or has cooled down enough to be safe. Second, be sure to place the beaker on the cork stand from the Starter Kit. Cork stands like these are designed to protect surfaces from high temperatures. Finally, if you need to move the beaker or stir the contents, only hold it using a towel or thick cloth.
Step-by-step instructions
You are going to produce quite a volatile compound in this experiment, so be sure to work in a well-ventilated area.

First, you'll need to dissolve some copper sulfate CuSO4 in hot water.
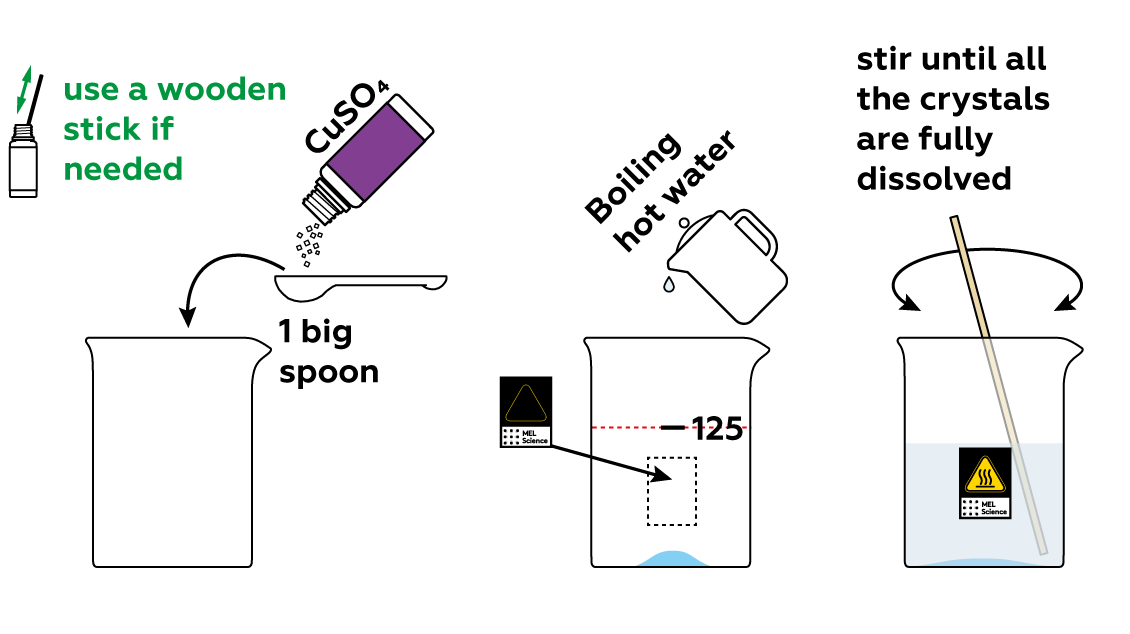
Immerse the various plastic objects in the copper sulfate CuSO4 solution. Then, add some potassium iodide KI to your solution.
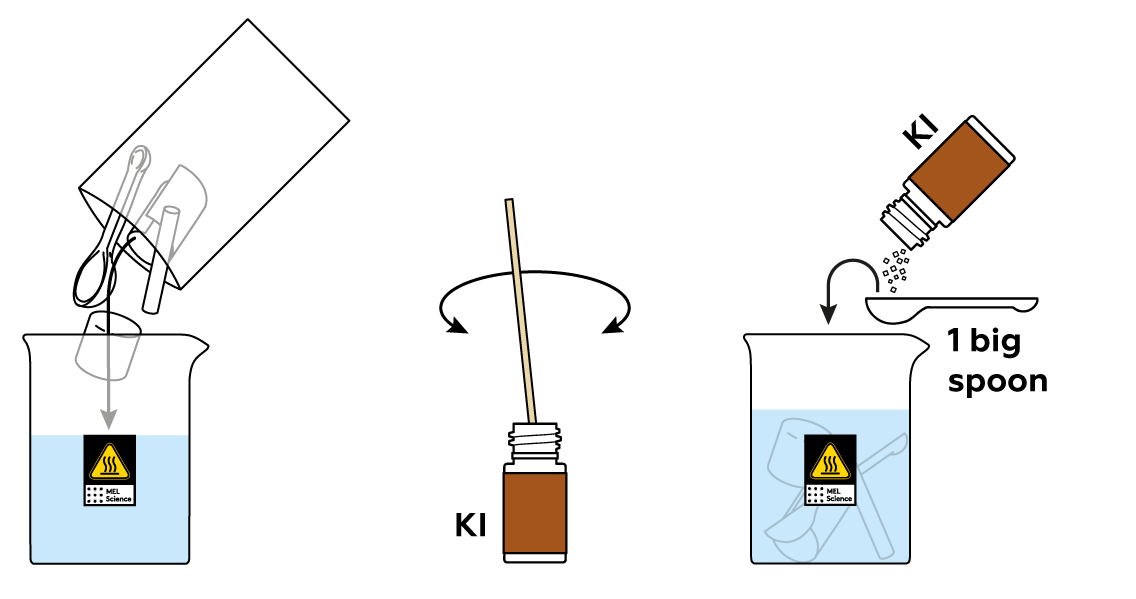
As you may notice, the solution immediately turns brown. You’re watching the I- from KI turn into iodine I2. Let the plastic objects sit in the solution for a while.
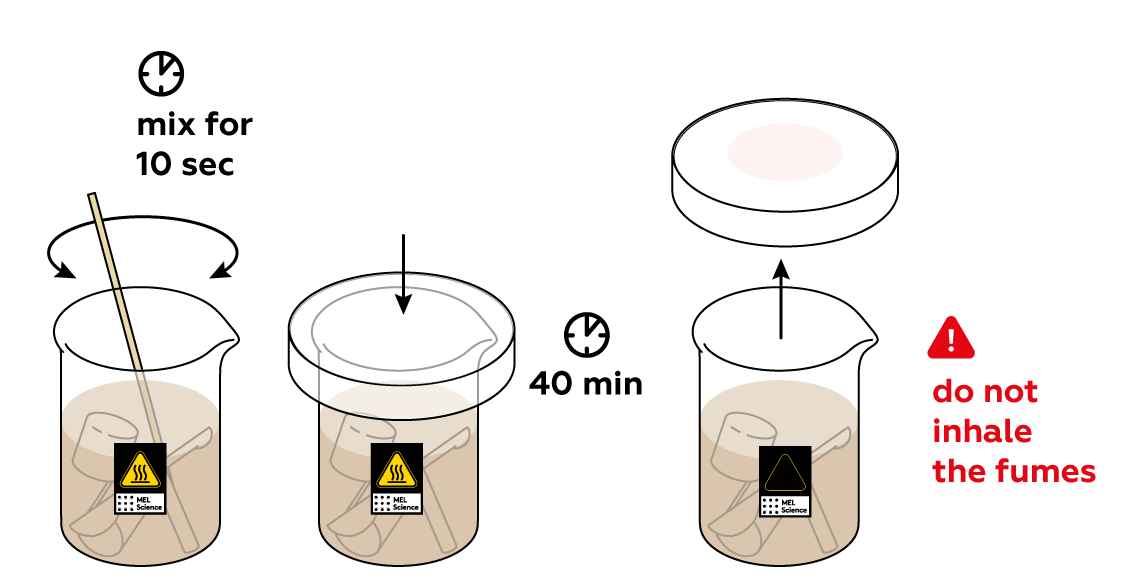
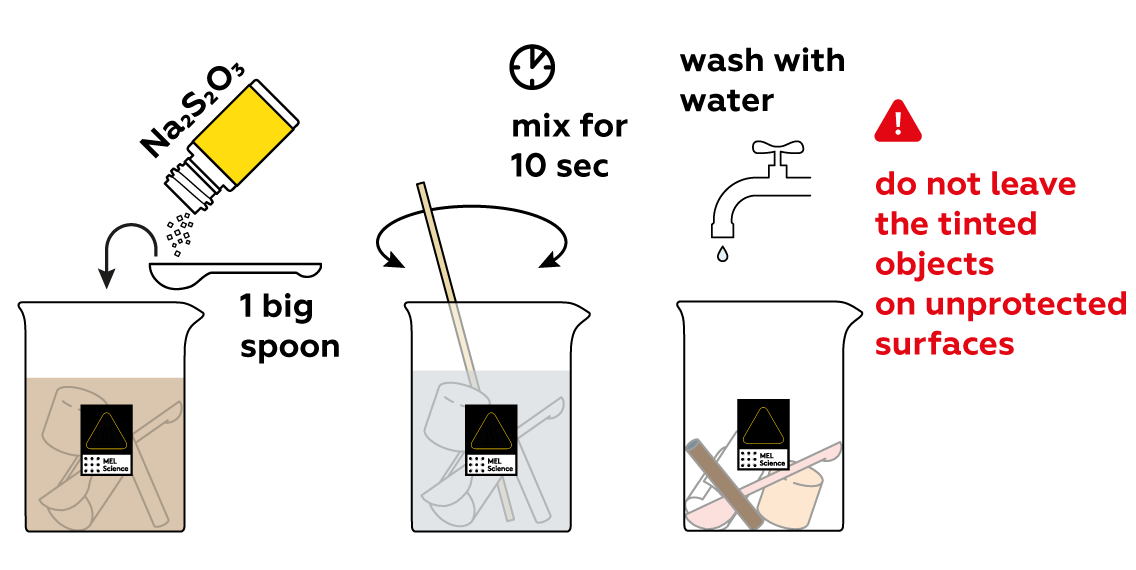
There's a lot of iodine I2 left in the solution. Turn it back into environmentally-harmless I- using Na2S2O3. You'll know that the reaction is done when the brown color disappears. Wash the plastic objects with water. As you can see, they changed colors after soaking in your iodine solution. Why?
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
When pure, iodine forms dark-purple crystals which easily turn into purple vapor. The iodine we have, however, is dissolved in water and is more brownish than purple. In general, depending on its environment, iodine can have different colors, including shades of brown , pink
. Iodine is really good at penetrating into many materials, and most of the plastics that we use are no exception. Iodine molecules easily pass in between the molecules of the plastics and thus stain the pieces all the way through. The plastics, in turn, provide unique environments for the iodine, just like air or water, and make the iodine brownish or pinkish in color.
That’s interesting!
Why doesn’t the Teflon film change colors?
Teflon actually is a brand name; the substance itself is called polytetrafluoroethylene. It is known for being one of the most inert compounds there is. This means that it doesn't react with many compounds, and is resistant to acids and bases. Its molecular bonds are so strong that the Teflon doesn't change colors during the experiment!
Fluoropolymer products don't burn, and due to this property, Teflon is often used as a coating for pans and irons. It’s also utilized in pipeline production, the automotive industry, and even in the production of clothing!