Liquid nightlight
Learn how to obtain light from liquid!
Safety
- Carefully review the general safety advice on the back of the box cover before starting the experiment.
- Be very careful in a dark room – turn the lights off only when necessary; be sure to prepare everything you’ll need in the darkness in advance; clear the path from the light switch to the table or, even better, ask someone else to turn the lights off.
- Never eat or drink any of the substances provided. Do not use for culinary purposes.
- Disassemble the setup after the experiment.
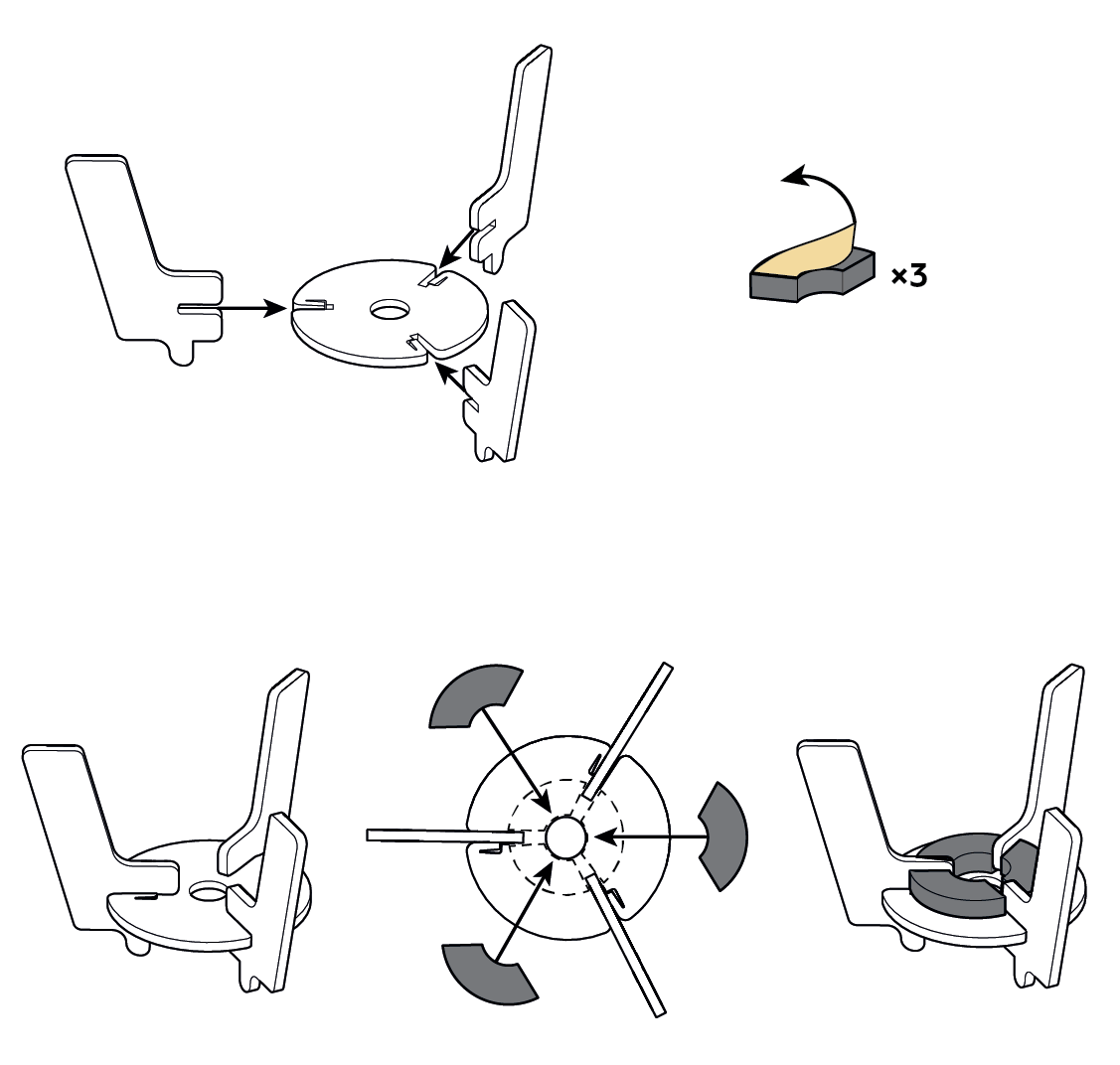
Step-by-step instructions
You can use a fluorescent glow to make your own nightlight!
Your UV light can promote fluorescence in certain substances.
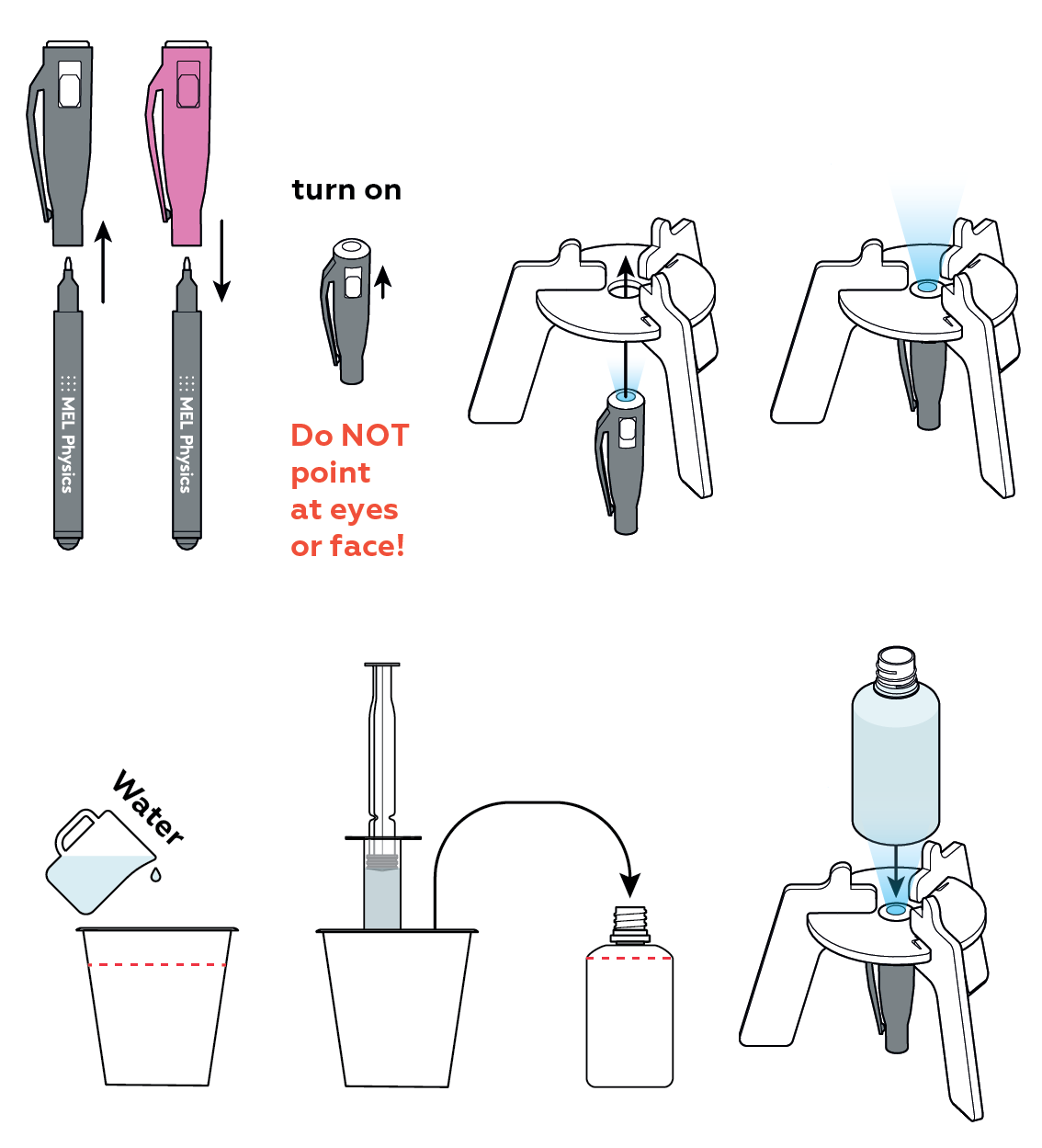
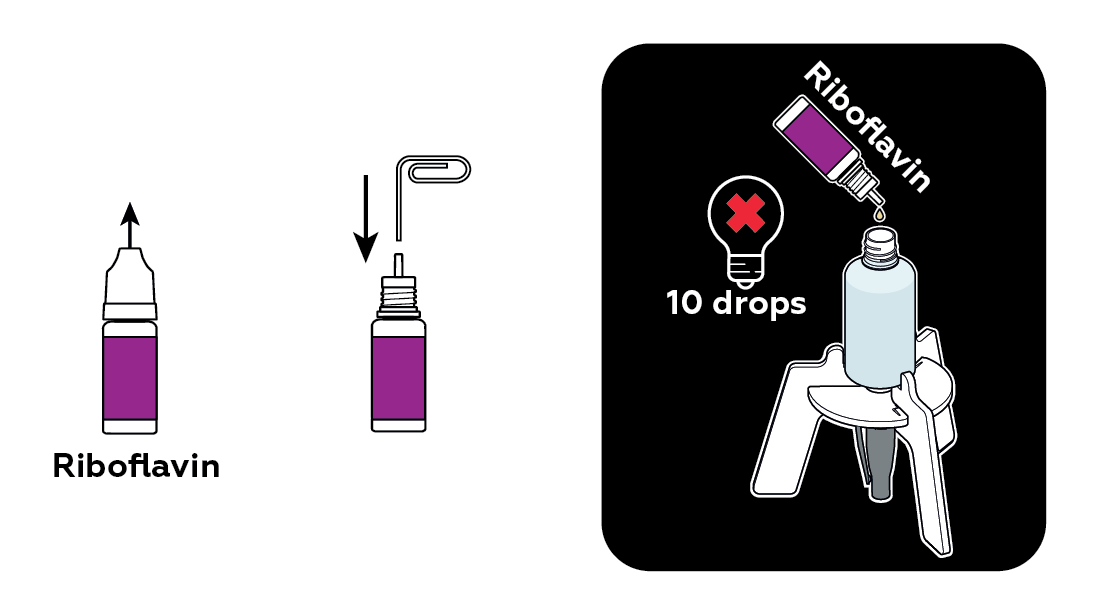
The dye you add to the bottle begins to dissolve. As it luminesces, you can see how the dye mixes with the water.
When the two liquids are completely mixed, your luminescent nightlight should glow uniformly. Enjoy!
Disposal
- Dispose of solid waste together with household garbage.
- Pour liquids down the sink. Wash with an excess of water.
Scientific description
Even individual photons have colors; the color of any light you see is actually the color of the photons
it consists of. As you may remember, when a molecule
absorbs a photon
, it gets some energy
. The amount of energy
the molecule
gets from a photon
varies depending on the photon’s color. A violet photon
will give the molecule
more energy
than a red one
. Simply put, the photons themselves have energy, and photons of different colors have different amounts of energy. Look at the picture!
As you move from left to right on this scale, the photons depicted carry progressively more energy (“weak” on the left, “strong” on the right). The color of the light produced also changes from left to right, from infrared through the visible colors in a rainbow order to ultraviolet.
Besides photons that form visible light, there are photons invisible to the human eye. But some molecules
can absorb these invisible photons and take their energy
. Photons that have more energy
than violet photons
are referred to as ultraviolet, and those that have less energy
than red photons
are known as infrared.
In the process of fluorescence, molecules absorb and then produce photons
. But why do we use a special UV light to initiate fluorescence? The light produced by the UV light has more violet and ultraviolet photons than daylight or lamplight. This is important because molecules can only emit photons with less energy than the photons they absorb. Shining violet and ultraviolet photons on riboflavin produces a green glow, which consists of photons with less energy. Consequently, it would be pointless to try to induce the fluorescence of riboflavin with a red flashlight, because red photons cannot supply molecules with enough energy to create a green glow.