Characteristics and properties of sodium
What properties are typical for sodium?

Sodium is an element in the 1ˢᵗ group of the 3ʳᵈ period of the Periodic Table. It is in the subgroup of alkaline metals; Na₂O sodium oxide and NaOH sodium hydroxide display typical base properties. The most important natural compounds of sodium are table salt NaCl, Glauber’s salt Na₂SO₄·10H₂O and sylvanite NaCl·KCl.
Physical properties
Like other alkaline metals, sodium is a silvery-white metal. It is quite malleable and soft (metallic sodium can easily be cut with a knife or scalpel, and a fresh cut shines in air). It has high electroconductivity.
Obtaining sodium
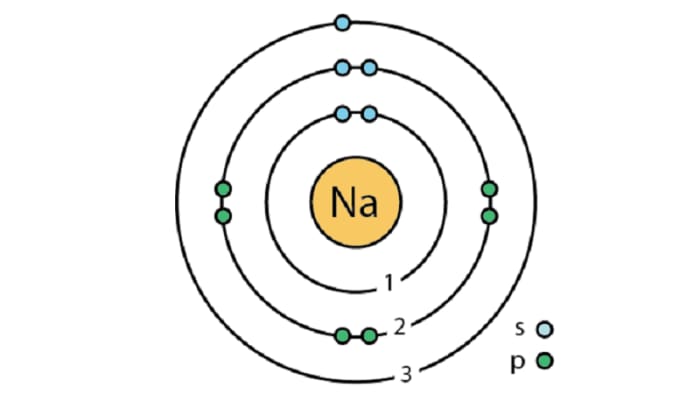
There are several methods for obtaining metallic sodium:
- electrolysis of a flux of sodium chloride:
К(-): Na⁺ + 1e = Na⁰;
A(+): 2Cl⁻ – 2e = Cl₂⁰.
2NaCl = 2Na + Cl₂.
- thermal decomposition of sodium azide:
2NaN₃ = 2Na + 3N₂ (that salt is heated to 250-300 ᵒC or 482-572 ᵒF);
- reduction of sodium carbonate (the first known method of obtaining metallic sodium):
Na₂CO₃ + 2C = 2Na + 3CO (reaction takes place with heating to 1000 ᵒC (1832 ᵒF)).
At present, the main method for obtaining sodium is electrolysis of fluxes of its salts (for example NaCl).
Chemical properties of metallic sodium
Metallic sodium is usually stored under a layer of kerosene, as it has high chemical reactivity (Na can react violently with oxygen and air moisture even at room temperature). The presence of sodium ions in a salt can be determined by the color of a flame – Na⁺ turns flame yellow.

Reaction of sodium with oxygen
When sodium burns in oxygen, the main product of reaction will not be sodium oxide, but sodium peroxide:
2Na + O₂ = Na₂O₂ (sodium oxide Na₂O also forms in this reaction, but in trace quantities).
In air, sodium can also swiftly oxidize to sodium oxide (the oxide will not be pure, as a considerable (sometimes greater) quantity of peroxide Na₂O₂ will be present among the products):
4Na + O₂ = 2Na₂O.
The reaction of sodium with oxygen may be written as follows:
6Na + 2O₂ = Na₂O₂ + 2Na₂O.
Reaction of sodium with non-metals
Sodium, besides combustion, is capable of taking part in many other reactions with non-metals:
- reaction with hydrogen with formation of hydride:
2Na + H₂ = 2NaH (reaction takes place with heating to 250-400 ᵒC (482-752 ᵒF) and at increased pressure);
- reaction with sulfur with formation of sulfides:
2Na + S = Na₂S (sodium sulfide);

- reaction with halogens:
2Na + Cl₂ = 2NaCl (sodium chloride);
2Na + Br₂ = 2NaBr (sodium bromide);
Metallic sodium reacts poorly with nitrogen (the reaction may be conducted in a glow discharge – a burning discharge formed in a low current and with low gas pressure):
6Na + N₂ = 2Na₃N.
Reaction of sodium with metals
When the surface of metallic sodium contacts mercury, an amalgam is formed – an alloy of metal with mercury.
With potassium, the metal forms an alloy with the formula NaK. It is quite aggressive – it may combust in air. If the content of potassium in the alloy varies from 40% to 90% in relation to sodium, the alloy remains liquid at room temperature. The sodium-potassium alloy NaK is obtained by alloying liquid potassium hydroxide KOH with melted metallic sodium. This reaction is carried out at a temperature of 400-450 ᵒC (752-842 ᵒF).
Reaction of sodium with compounds
Owing to its chemical reactivity, sodium may enter into reactions with various compounds, for example:
- reaction of sodium with acids (diluted):
2Na + 2HCl = 2NaCl + H₂ (sodium chloride NaCl and hydrogen gas H₂ form).
- reaction with nitric and sulfuric acids:
8Na + 8H₂SO₄ = NaHS + 7NaHSO₄ + 4H₂O (with heating, concentrated acid);
8Na + 10HNO₃ = 8NaNO₃ + NH₄NO₃ + 3H₂O (diluted acid – 3-5%);
11Na + 14HNO₃ = 11NaNO₃ + NO + N₂O + 7H₂O (acid of 20% concentration).
- reaction with water (reaction takes place vigorously):
2Na + H₂O = 2NaOH + H₂.

- reaction with sodium peroxide:
2Na + Na₂O₂ = 2Na₂O.
With ammonia 2 types of reaction are possible:
- reaction of sodium with ammonium gas:
2Na + 2NH₃ = 2NaNH₂ + H₂ (sodium amide forms with heating to 350 ᵒC, or 662 ᵒF);
- dissolving sodium in liquid ammonium:
Na + 4NH₃ = Na[NH₃]₄ (reaction with formation of tetra sodium amide takes place in cold – cooling to -40 ᵒC (also -40 ᵒF)).
Here you’ll find an interesting experiment with ammonia solution.
Reactions of sodium with organic materials
Sodium has also found an application in organic chemistry – it is used to obtain alcoholates from alcohols:
2C₂H₅OH + 2Na = 2C₂H₅ONa + H₂ (from absolute (waterless) ethanol, sodium ethylate C₂H₅ONa forms).
Sodium is also used to extend carbohydrate chains in the Wurtz reaction:
CH₃-CH₂-Br + Br-CH₂-CH₃ + 2Na = CH₃-CH₂-CH₂-CH₃ + 2NaBr.
Sodium breaks bromine atoms off molecules of bromine ethyl. The “connection” of formed carbohydrate radicals takes place with formation of a longer molecule – the two ethyl radicals CH₃-CH₂- connect, forming the n-butane molecule CH₃-CH₂-CH₂-CH₃).
Sodium is often used as a reducer in metallurgy and a drying agent of organic solvents (for example esters) in organic synthesis. The metal is also used for the manufacture of high-capacity batteries.