Calcium silicate
Obtain a self-solidifying mixture!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Is this normal?
Yes, this is exactly as it should be. Calcium hydroxide is only slightly soluble in water. Dissolving the given volume of calcium hydroxide in its entirety would require almost 2 liters of water! Thankfully, this experiment only requires that you mix calcium hydroxide thoroughly with water, not that you make it dissolve completely.
When you mix the contents of the two cups, you are creating the mixture that will eventually solidify. This transition from one state into another is easiest to observe if you keep pouring the mixture. This is also why you should first pour the liquid glass into a separate cup. Adding it directly to the calcium hydroxide would cause the mixture to solidify too soon.
Perhaps the calcium hydroxide and water weren’t mixed thoroughly enough. This can cause the reaction to happen unevenly or with a significant delay. Try mixing the contents more thoroughly or repeating the experiment.
Step-by-step instructions
First, prepare two cups: one with sodium silicate solution (also known as liquid glass), and one with calcium hydroxide solution.
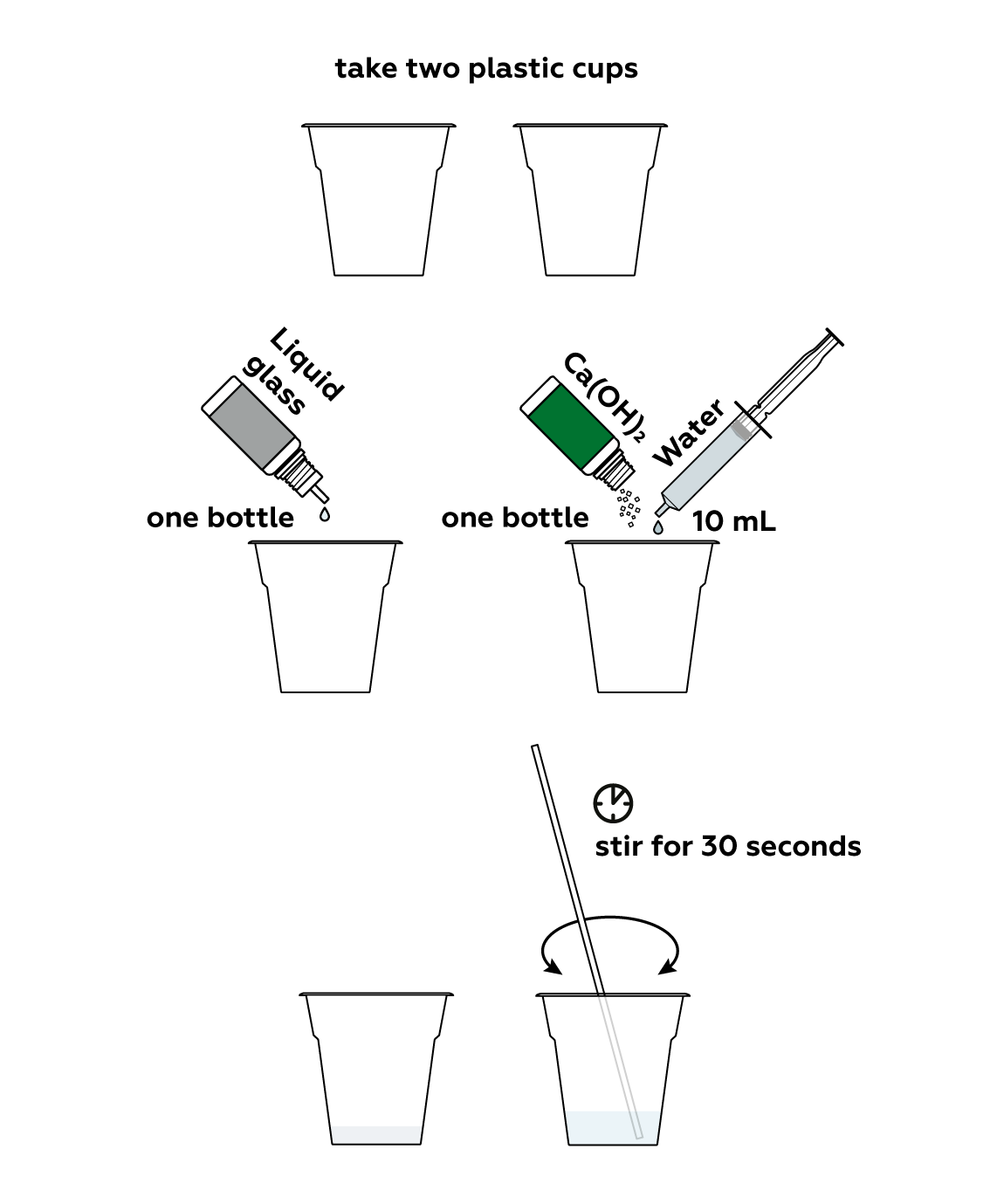
At first, when the two liquids are mixed, it would seem that nothing is happening...
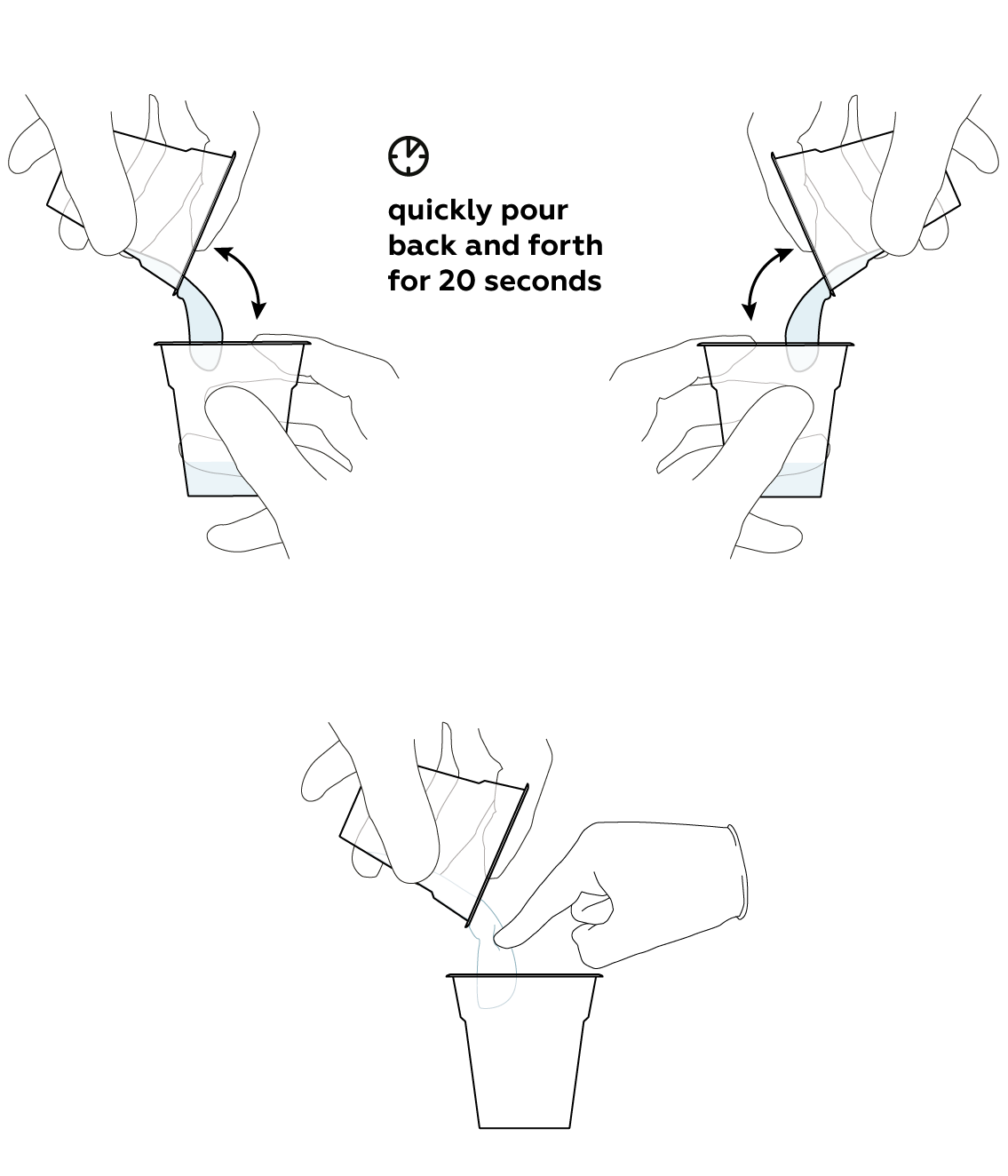
Expected result
Calcium hydroxide Ca(OH)2 reacts with sodium silicate Na2Si2O3 and this provides the insoluble calcium silicate CaO*SiO3. This is why the solution hardens right in the air! By the way, calcium silicate is a rather popular material for modern day industry. E.g. it is used for passive fire protection and in some types of cement.
Disposal
Dispose of solid waste together with household garbage.
Scientific description




which is almost completely insoluble. Normally, this compound looks like a boring white powder.




What is the substance we obtained?
The material produced in this experiment can be called a plastic.
Plastics are typically organic substances and contain a significant proportion of carbon and hydrogen atoms bonded together. However, the plastic in this experiment is of a different nature: there is no carbon in it at all. It is made of silicon Si, oxygen O, sodium Na, and calcium Ca. The key reaction used to produce this material is the combination of silicon and oxygen atoms, a combination that can often be found in silicates. Since silicates are inorganic substances, such plastics are called inorganic or silicate plastics.
What is liquid glass?
Liquid glass consists mostly of sodium silicate Na2SiO3 and a few other, similar sodium silicates, the only variable amongst them being the proportions between sodium Na, silicon Si, and oxygen O atoms. Liquid glass is actually a stable form of the sodium silicate solution in water.
Liquid glass is an inorganic polymer. Polymer molecules are very big but relatively simple, consisting of a large number of repeating fragments (monomers) connected one to another via chemical bonds. You can picture a polymer as a chain consisting of a number of identical elements.
Liquid glass, however, is an anionic polymer. When dissolved in water, anionic polymers form long, negatively-charged chains with a corresponding number of positively charged ions surrounding them in the solution. Liquid glass specifically (which is actually sodium silicate Na2SiO3) consists of polymeric silicate anions (-Si-O-Si-O-) with Na+ ions floating around them in the solution.
Why does the reaction mixture solidify?
The mixture solidifies due to a chemical reaction between its two main components: calcium hydroxide Ca(OH)2 and liquid glass (Na2SiO3). Together, they form a solid material that is insoluble in water. This material strongly resembles the common silicate glass used in windows, mirrors, drinking glasses, etc., which is made of bonded calcium, sodium, and silicate ions.
The nature of the calcium hydroxide Ca(OH)2 determines the outcome. If we add another source of calcium (for example - CaCl2), we will create a solid, crackly material that bears a strong resemblance to sand.
There aren’t many calcium Ca2+ ions in the solution. Compared to sodium Na or potassium K hydroxides, calcium hydroxide is far less soluble in water. When it dissociates in water, very few calcium ions Ca2+ enter the solution:
Ca(OH)2 ↔ Ca2+ + 2OH–
This is why the material forms relatively slowly and all over the solution simultaneously.
At the beginning of this process, tiny but constantly-growing bits of insoluble calcium silicate form. Gradually, thin bridges materialize between these bits, connecting them in a cluster that gradually spreads all over the solution. These bridges are rather strong, so the resulting particle does not collapse. At the same time, the bridges are also flexible, so the resulting substance remains ductile for quite some time.
Try thinking of the flexibility of these bridges of glass-like material like this. It is impossible to bend a glass rod as thick as a pencil – it will break (but don’t try this at home – it’s dangerous!). However, it is possible to bend a glass rod if it is as thin as a thread. These bridges are even thinner, invisible to the naked eye, so they are much more flexible. Moreover, even if some of the bridges break due to excess pressure, this will not affect the network as a whole.
Water molecules also play a key role in the flexibility of the silicate plastic. They act as a temporary “airbag,” preventing the clusters from sticking to each other. These water molecules are actually trapped between these clusters of sodium silicate. They have no choice but to stay – the sodium silicate crystallizes all over the solution, soaking up all the liquid at once
Why does the material lose flexibility with time?
The substance dries as water evaporates from it. Dried calcium silicate resembles white sand.
Again, water is key. More specifically, the key is in how it evaporates. As the material gradually dries, water molecules no longer prevent the calcium silicate clusters from sticking to each other.
Also, the formation of the bridges we described above is not the end of the process. Calcium ions Ca2+ slowly enter the solution and firmly bind the growing clusters of material to one another. This is why the properties of silicate plastic become similar to those of a glass rod, which would sooner break than bend.
Can we reverse the drying process and make the material flexible again?
No, we can’t. There is no way to recreate this material starting with sand-like calcium silicate. This material was ductile partially due to the way we obtained it from the initial reagents.