Isotopes
Students will learn that different carbon atoms always contain 6 protons but can contain different numbers of neutrons. These different atoms are called isotopes. This lesson will reinforce understanding of element symbol notation.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
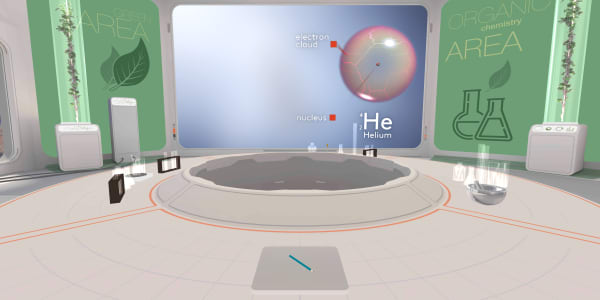
We have already learned that an atom consists of a small heavy nucleus surrounded by electrons spread out in space. Today, we will learn more about the structure of a nucleus.
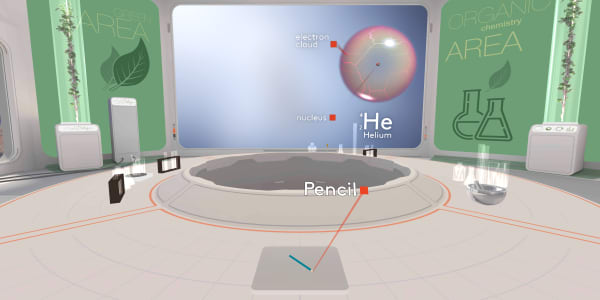
Let's take our pencil and look inside. Ready to dive?
We have to zoom in a billion times to see the individual carbon atoms.

Now let's choose one of those atoms and get closer to it.
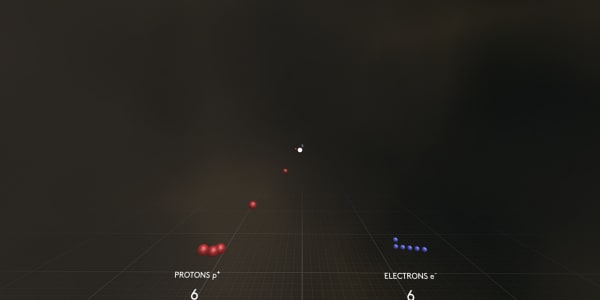
Let's disassemble our carbon atom to see what it is made of.
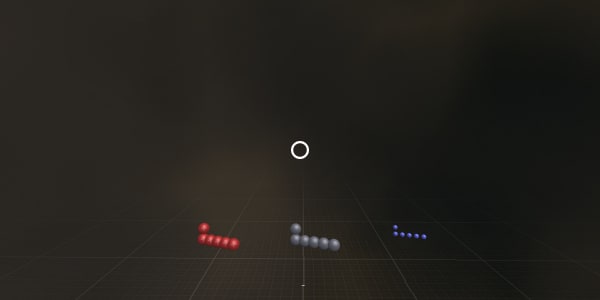
Please point – where are the protons?
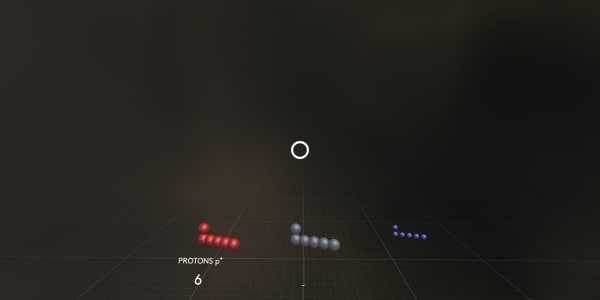
Protons are positively charged particles from the atom’s nucleus.
Now where are the electrons?
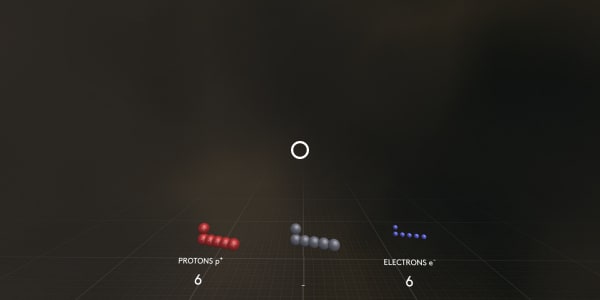
Electrons are negatively charged particles over a thousand times lighter than protons or neutrons.
Finally, where are the neutrons?
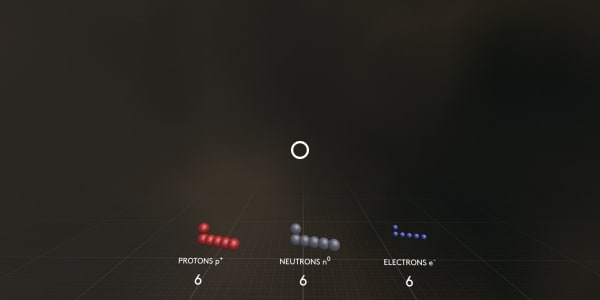
Neutrons have almost the same masses as protons, but they are not charged.
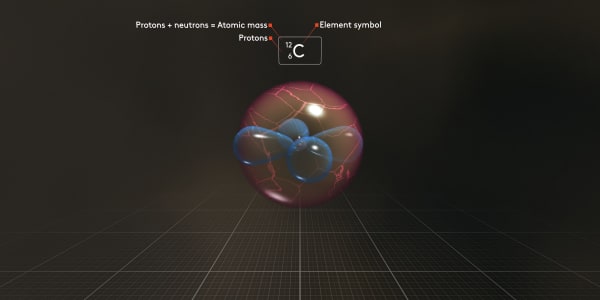
We use symbol the C_6_12 for this atom to show that it has 6 protons and its total atomic mass is 12, which is the number of protons and neutrons combined.

The number of protons, 6, is what makes this atom a carbon atom. This is called the atomic number.
Let's look at another carbon atom.
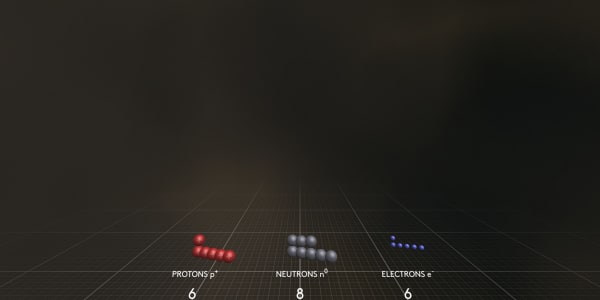
As you can see this carbon atom contains 6 electrons, 6 protons and 8 neutrons.
Atoms with the same number of protons but a different number of neutrons are called isotopes.
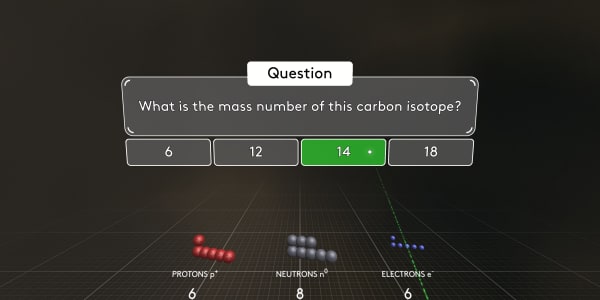
What is the mass number of this carbon isotope?
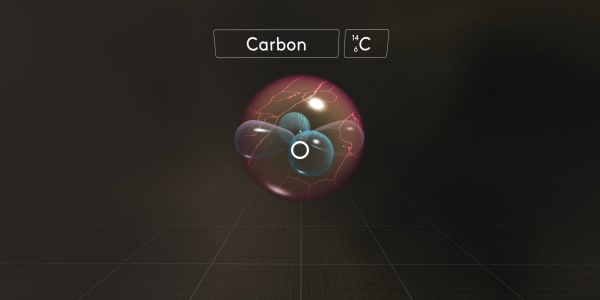
As you know, protons and neutrons have almost the same mass. And electrons are much, much lighter. So the atomic mass number is the number of protons and neutrons combined. In this case, the atomic mass number is 14.
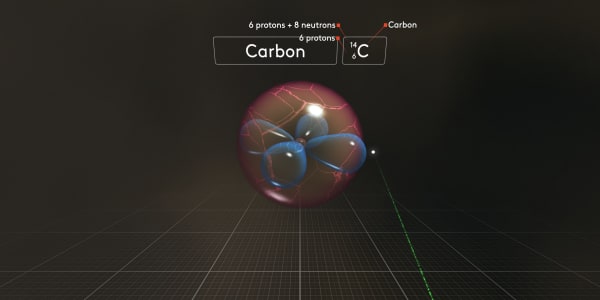
For this isotope, we would use the symbol C_6_14, where 6 is the number of protons and 14 is the total atomic mass.
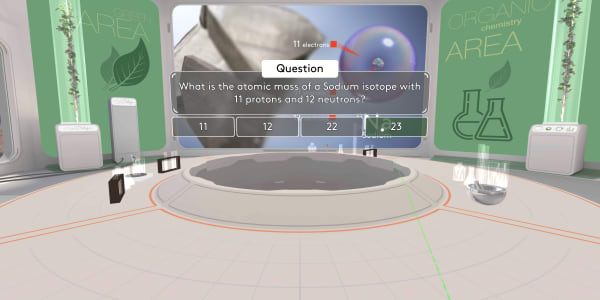
Now, let's go back to our laboratory.
What is the atomic mass of a Sodium isotope with 11 protons and 12 neutrons?

The atomic mass number is the number of protons and neutrons combined. Eleven plus twelve is 23.
Teacher's notes
Keywords
atoms, electrons, nucleus, protons, neutrons, isotopes, mass number
Common misconceptions
- Isotopes are different elements because their nuclei are different.
- Confusion between mass number, atomic number, and atomic mass.
Students will
- Recall that an atom’s nucleus consists of positively-charged protons and uncharged neutrons
- Learn that protons and neutrons are much heavier than electrons
- Learn to interpret element notations, e.g. 126C, 146C
- See that atoms of the same element can contain different numbers of neutrons in their nuclei
- Learn how to calculate an atom’s mass number
Hands-on activities
After VR
Discussion of half-life concept.
Explore how radioactive isotopes decrease in quantity over time.
Students are given a cup of small grains (like rice) and are instructed to remove half of the remaining amount every 30 seconds.
History and sources of knowledge
- Soddy’s discovery of radioactive isotopes.
- Thomson’s discovery of stable isotopes.
- Modern methods of mass-spectrometry showing isotopic composition.
- Modern separation methods.
Topics to discuss
- Half-life concept.
- Why the number of neutrons in an atom does not affect its chemical properties.
- Atomic mass as average mass of different isotopes.
Fun facts and quotes
- You can accumulate heavy water (water that contains deuterium, a hydrogen isotope with one proton and one neutron) in your kitchen, if you don't replace the water in your kettle for a very long time and add more water as the water in the kettle evaporates.
- An isotopic analysis can not only tell you when organisms lived, but also what they ate and their migration paths.
- All synthetic elements only have unstable (radioactive) isotopes.
- Radiocarbon dating is used to determine the ages of once-living organisms (from millions of years old to thousands of years old).
- The origin of wine can be easily determined by the ratio of stable isotopes of the different elements in the wine.
Questions
- What distinguishes the atoms of one element from the atoms of another?
- Two atoms have the same number of neutrons but a different number of protons. Are they isotopes?
- Two atoms have the same number of protons but a different number of neutrons. Are they isotopes?
- What does the number 7 represent in the isotope 7Li?
- List the number of protons, neutrons, and electrons in this pair of isotopes: 4020Ca/4420Ca.
Calculating
- Calculate the number of neutrons in the different isotopes of oxygen.
- Calculate the atomic mass from the percentage and mass of two isotopes (for example, for 35Cl/37Cl).
- Calculate the isotopic ratio (in percentage) from the mass-spectrum (for example, 63Cu/65Cu).
Quiz
Please see below for the link to a Google form containing a quiz on the material above.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.