Rainbow plating
Capture a rainbow on a metal plate!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
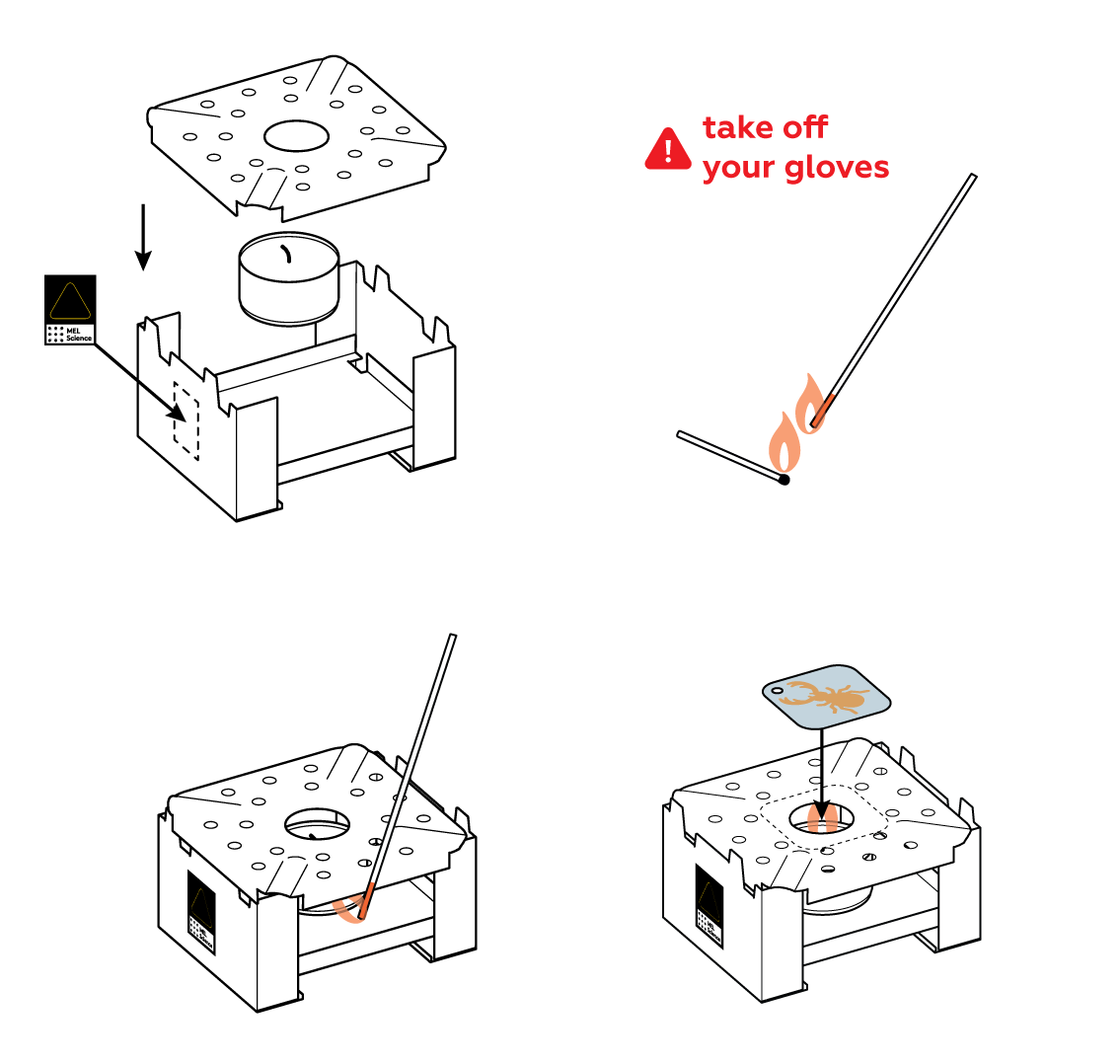
- Remove protective gloves before lighting the splint.
- Keep flammable materials and hair away from flame.
- Keep a bowl of water nearby while working with fire.
- Do not touch the stove after the experiment – wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
This set contains two types of plates: iron and copper. The copper plates have a characteristic orange color. The iron plates are grey.
The shinier the surface, the better. There shouldn't be any streaks, rust, or patches.
Traces of oils from your fingers (even recently washed) can remain on the metal. The reaction will not occur in these areas, and it can spoil the result.
No, you’ll have to polish the plate again. Any glue particles left on the metal can interfere with the experiment.
Remove the sticker, polish the back of the plate, and apply the sticker outline to the back. Continue the experiment with this side of the plate.
If you plan to repeat the experiment, first make sure that the iron plate is very well polished (it should be shiny). Second, briefly apply the solution of CuSO4 to the iron plate. It usually takes no more than ten seconds for the copper layer to appear. Finally, immediately rinse the plate with water as soon as the copper layer appears.
You probably overexposed the plate to the CuSO4 solution, causing the copper layer to become too thick. Unfortunately, you’ll have to start over. After you apply the copper sulfate CuSO4 solution to the plate, watch it closely and rinse it with water as soon as the copper layer appears. And use gentler running water.
You probably overexposed the plate to the CuSO4 solution, causing the copper layer to become too thick. Unfortunately, you’ll have to start over. After you apply the copper sulfate CuSO4 solution to the plate, watch it closely and rinse it with water as soon as the copper layer appears.
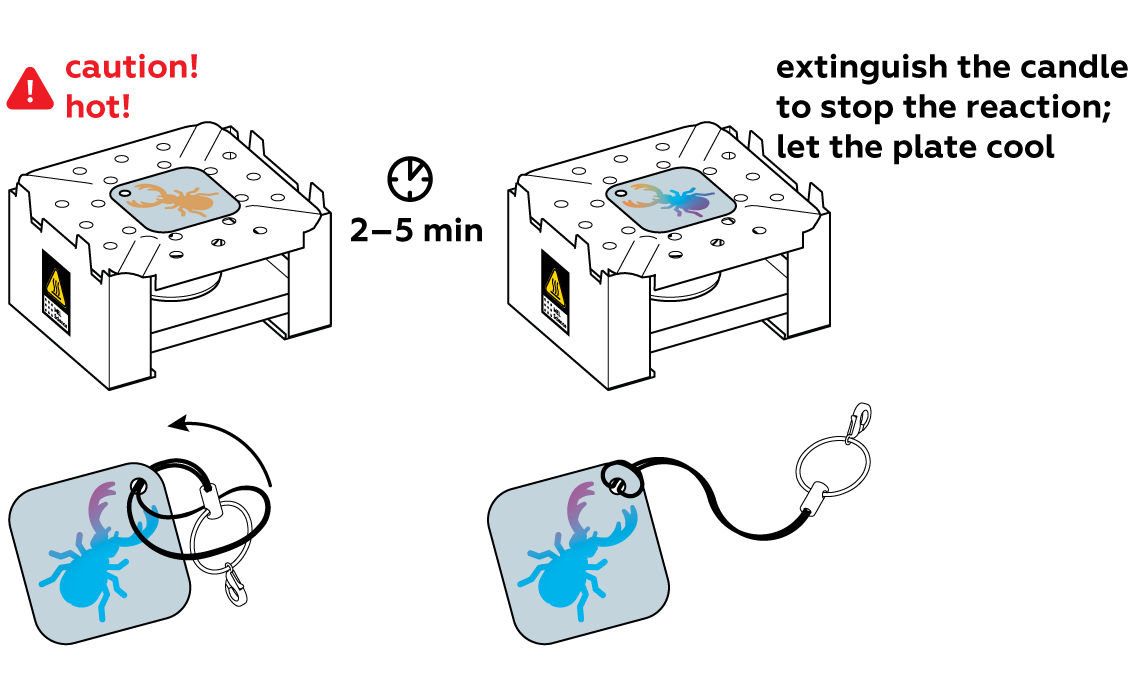
This is normal! Copper transitions orange – pink – purple – blue – light blue – white. If your coating turned white, then you passed all these stages! If you are not satisfied with the color you got, try again and extinguish the candle when you get the color you want.
We recommend coating the keychain with a clear varnish or hanging it where it won’t be subjected to friction.
Step-by-step instructions
Clean the surface of the iron so that it can easily engage in chemical reactions.
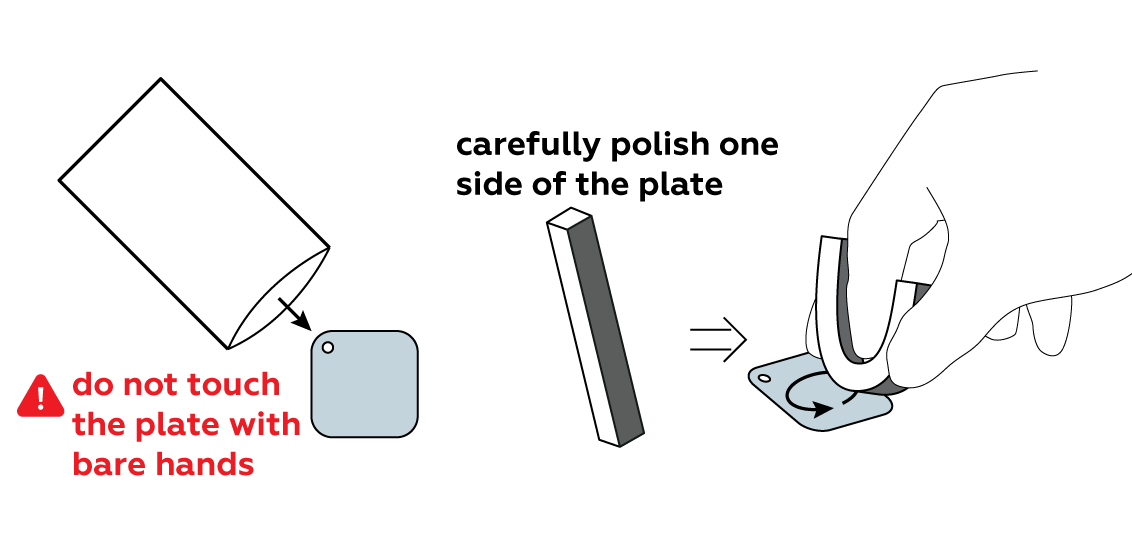
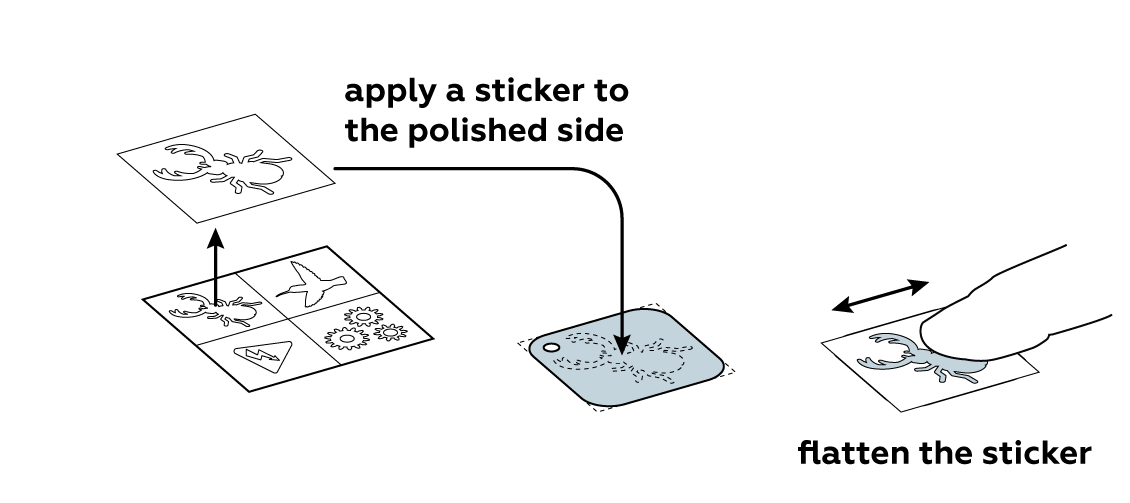
Protect part of the iron plate with a sticker of your choice.
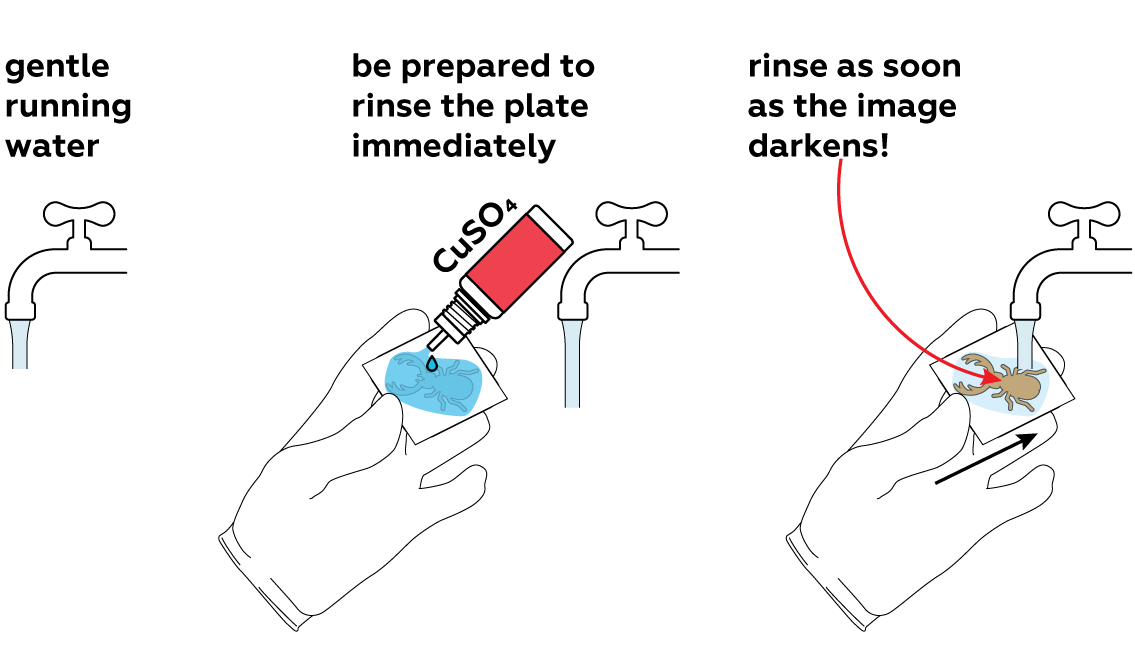
Generously apply copper sulfate CuSO4 solution to the unprotected section of the plate and observe the reaction. For the best results, rinse it with water as soon as the surface darkens. This will stop the reaction.
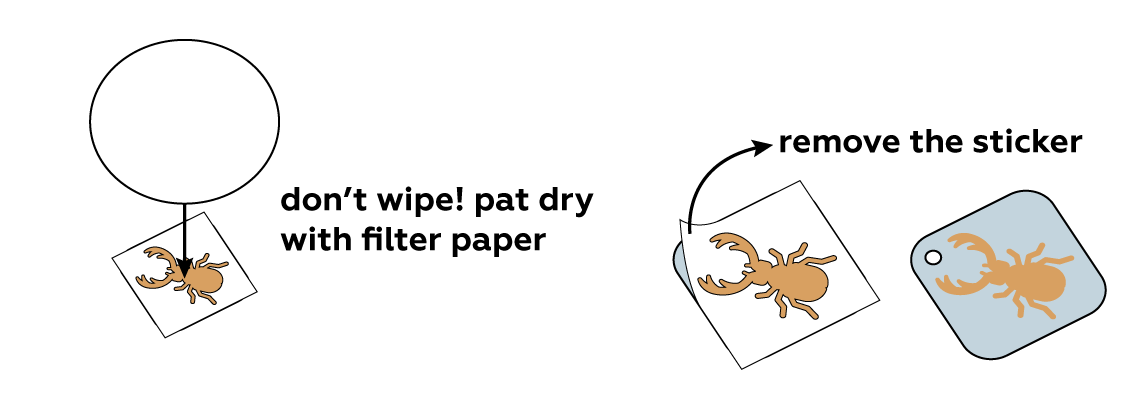
Carefully pat the plate dry and remove the sticker. You can already see the image—but wait, there's more!
The image you see on the iron plate is formed by a thin layer of copper from the copper sulfate. When heated, copper can react with oxygen in the air to form copper oxides.
Copper oxides are usually just boring powders, but they look quite spectacular when they form as thin layers.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description








This optical property of extremely fine structures is cleverly employed by some living organisms. In nature, it's sometimes very tricky for an organism to produce a pigment of a certain color, say, blue. But an organism can use easily-obtainable materials of boring colors to form special "light traps" that only reflect certain colors, which usually look much more exciting. Many birds
and bugs
use this trick. Can you picture the green luster on some beetles, or even a blue jay's vibrant plumage? They possess no green or blue pigments—they trick our eyes with an optical illusion.
That’s interesting!
Where else can we see this rainbow effect?
Such a rainbow effect can be found in the thin films of oil, kerosene, and other organic compounds that you can sometimes spot on the surface of the water. A drop of petroleum in a puddle makes the surface shimmer with all the colors of the rainbow. Though it’s pretty harmful to the environment, it’s breathtaking to see. You can also observe a similar effect in iridescent soap bubbles – you can try this one out at home anytime. You can also examine colorful holograms, which are applied to goods to protect against counterfeiting. Plus, check out decorative materials such as iridescent fabrics, artificial pearls, automobile paints, and nail polishes.
But this effect can predominantly be found in wildlife: in birds’ plumage, in insects’ integumentary tissues, in the squama of sea and ocean reptiles. Moreover, the colors’ shades and brightness can change when the viewing angle changes. Can you picture how starlings’ feathers or ducks’ necks shimmer blue-green? In addition to the rainbow effect, nature sometimes adds a glossy or mirroring effect. The latter can be seen on many fish.
Another good example is brightly-colored peacock feathers. In fact, these colors are an illusion: their feathers are predominantly brown. But the feathers’ special structure distorts the light waves they reflect, generating breathtakingly beautiful illusions!