Sulfur Melt
Watch the transformations of the yellowest chemical element
Reagents
Safety
- It is not recommended to perform the experiment if you have asthma.
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray and in a well-ventilated area.
- Avoid inhaling sulfur vapors and sulfur dioxide from the aluminum cup.
- Remove protective gloves before lighting the splint and put them back on after blowing out the candle.
- Keep a bowl of water nearby when working with fire.
- Keep flammable materials and hair away from flame/the setup.
- Do not touch the stove after the experiment. Wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
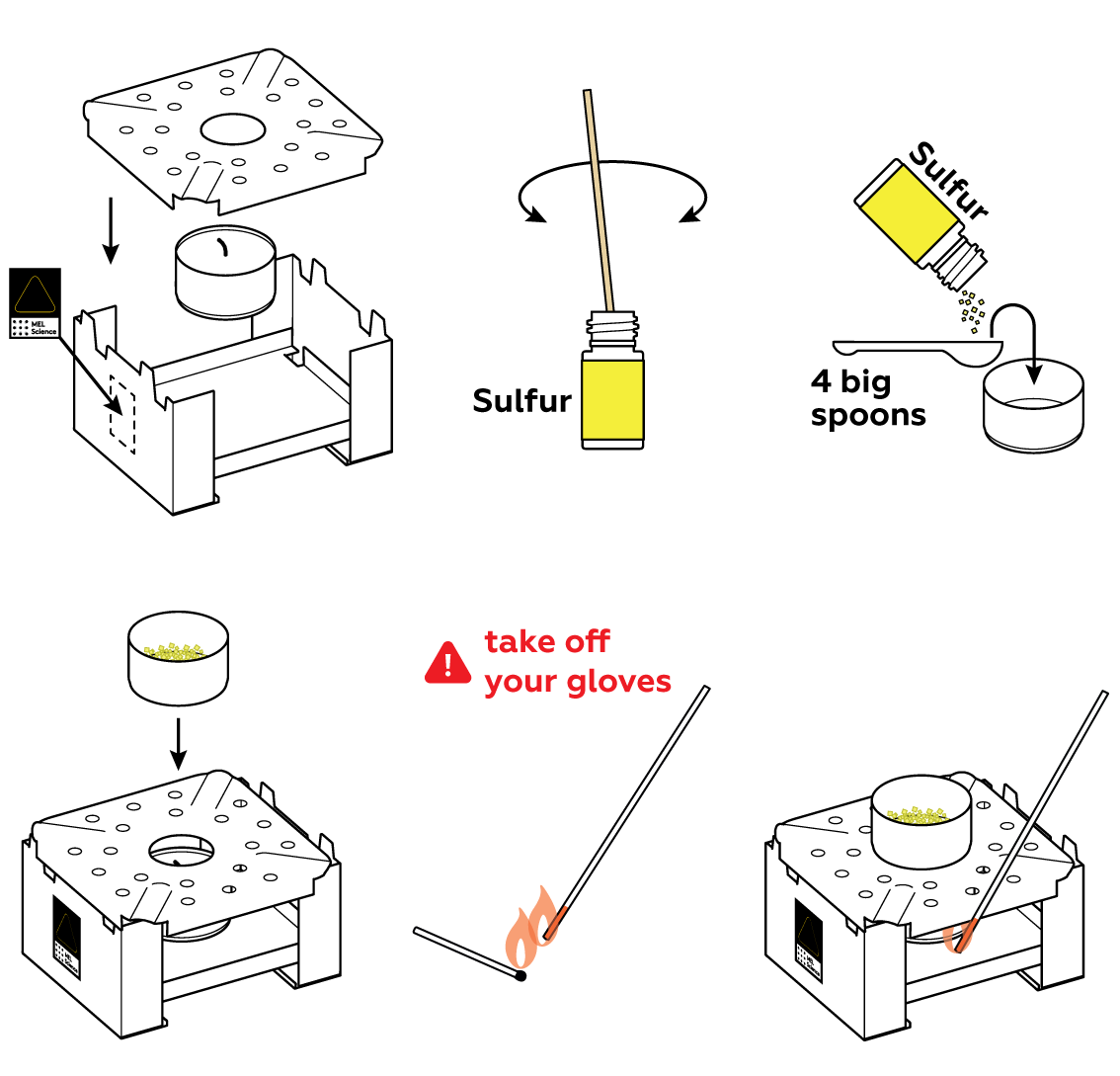
Step-by-step instructions
Sulfur is the fifth most common element by mass on Earth. It usually occurs in the form of complex compounds, some of which are essential for living organisms. You’ll hardly come across the yellow, powdery form you’ve got—its pure form—in everyday life. Let's study its properties.
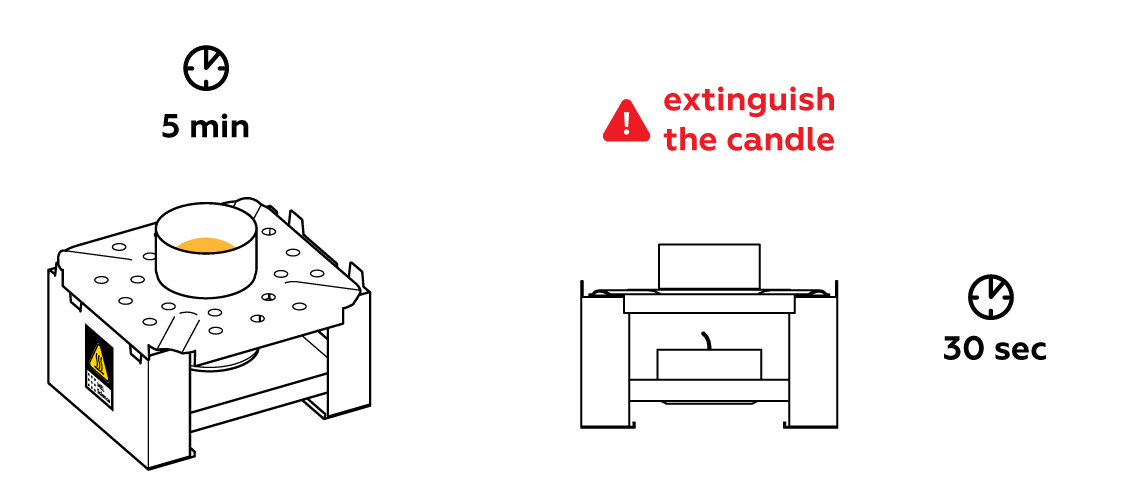
The molecules in solid sulfur occupy fixed positions. When heated, the molecules have a difficult time sitting still—they start to vibrate intensely and abandon their fixed “seats,” eventually so much that the solid turns into a liquid.
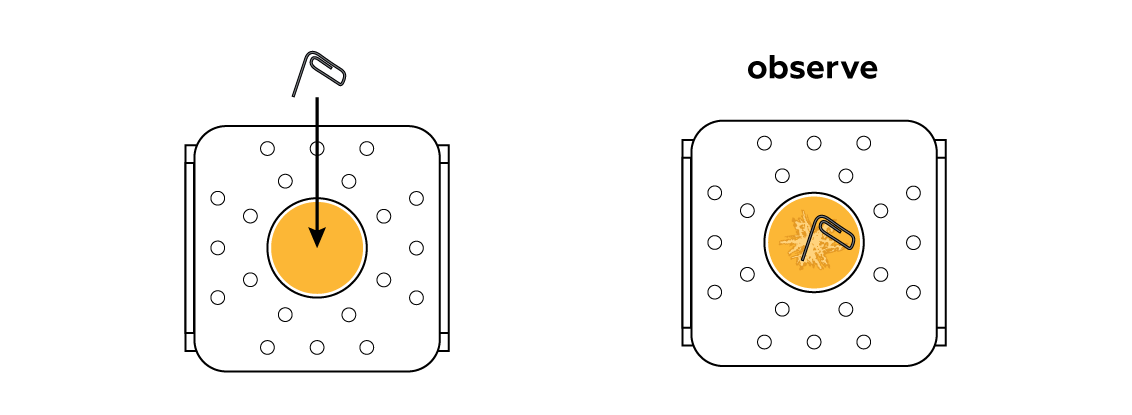
After the liquid sulfur cools down a little, its molecules slow down but don’t return to their seats just yet. The cold paperclip serves as a crystallization center: as the molecules bump into it, they settle down, forming a little sulfur crystal. Other nearby molecules run into this crystal and adhere to it one by one, expanding the crystal in all directions.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage.
Scientific description
Interestingly, the mobility of the molecules isn’t the only thing that changes in this experiment—their shape is altered, too.

are normally arranged in S8 molecules
that look like crinkled rings or crowns. These molecules are what form the basis of the yellow powder
.

and some of the rings break, forming chain molecules
that partly polymerize, meaning they combine with each other to form longer chains
: S16, S24, etc. When this happens, the liquid
acquires an orange tint.

coil back into S8 rings
. They arrange themselves differently than how they started, forming a dense structure resembling a puck
.
Can sulfur become malleable, like clay?
You’ve just seen what happens when liquid sulfur is heated for a short time and cooled. However, when heated for longer, liquid sulfur becomes reddish-brown and viscous due to the formation of much longer molecular chains . If this substance is cooled very quickly, its molecular chains
don't have time to break down. As a result, the substance turns into plastic sulfur and becomes malleable, like clay.