Magic liquid
Make water change colors!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
We recommend using the glass beaker for this experiment. But if you’ve already begun using the plastic beaker, don’t worry! Feel free to continue the experiment using it.
You likely didn't add enough water. If there is too little water and you don't mix the contents of the beaker, the solution may remain blue for some time. If you observe, you will see how the solution gradually changes colors. You can also mix the contents. For this experiment, you need to fill the beaker with water right up to the top of the line.
Don't worry! Pour the correct volume of solution into a second plastic cup. At the end of the experiment, compare the results between these two cups.
Mixing citric acid with sodium carbonate Na2CO3 produces carbon dioxide CO2, and this gas forms bubbles in the solution.
No problem – continue the experiment. At the end of the experiment – just like a real chemist! – you will be able to determine the composition of the solutions by their colors. If you want to repeat the experiment, you can number the plastic cups to help avoid confusion.
You can make a new solution of thymol blue using the same beaker and add the required amount to the remaining cups.
The color of the solutions depends on their pH, and their exact pH value can be influenced by various factors, such as the temperature or the concentration of reagents in the solution. Therefore, the solutions can have many different shades of color. You can expand on the experiment by making the solutions in the cups more saturated (adding more reagent) or increasing/decreasing the water temperature and observing how the color of the liquid changes. Also, try using clear, bottled, non-carbonated water. Tap water, carbonated water, or water with any sort of additives often has a non-neutral pH.
Step-by-step instructions
First, prepare a solution of a pH indicator thymol blue.
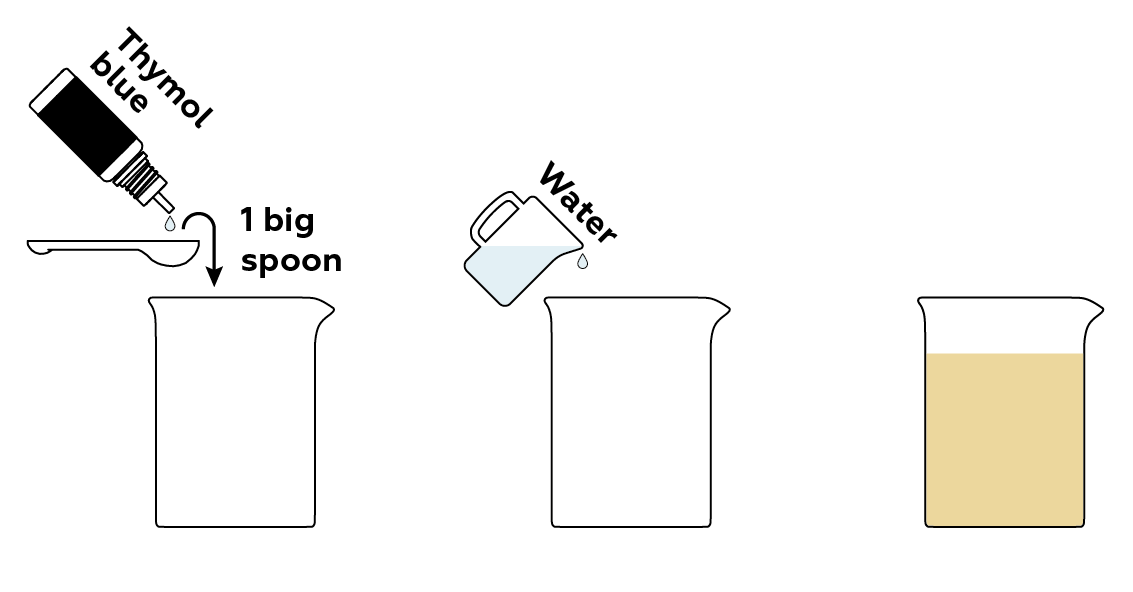
Now, prepare samples of compounds that will influence the solution’s pH in different ways.
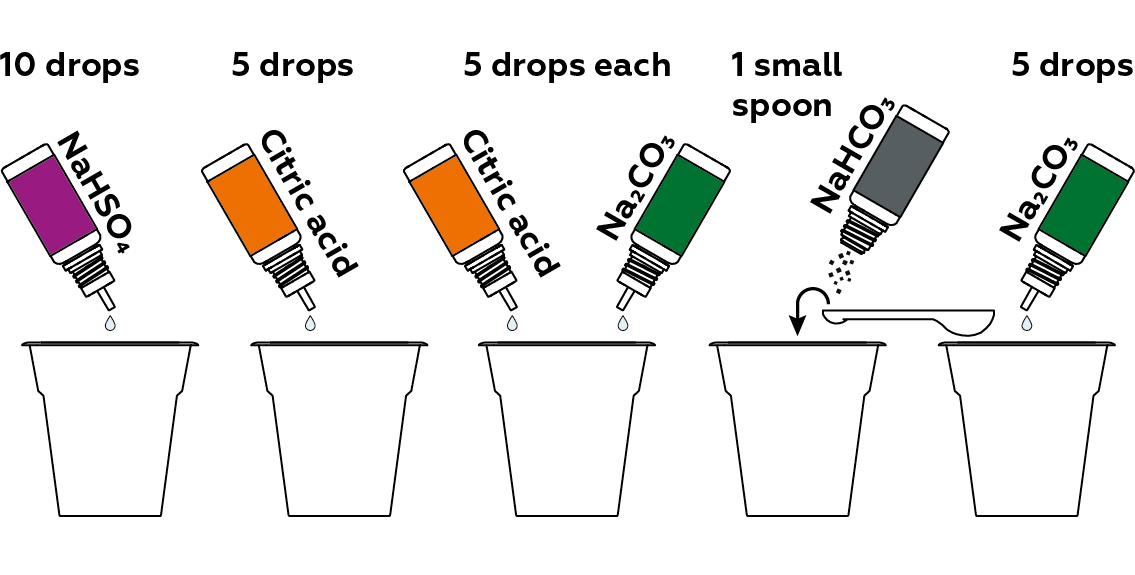
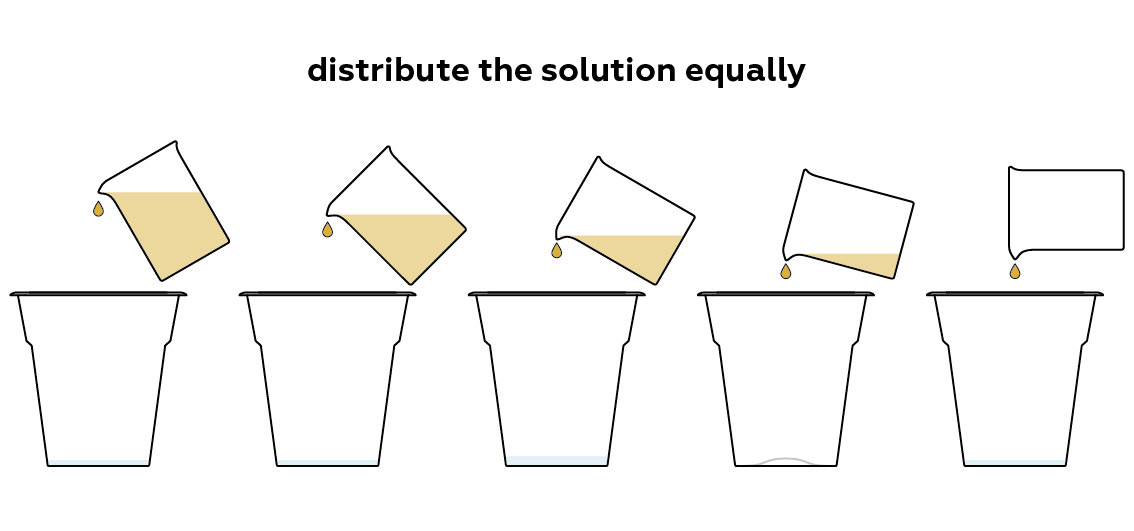
Add the pH indicator solution to each of the samples.
pH indicators are substances which, when added to a solution, change color in accordance with the solution’s pH. One can determine a solution’s pH by the indicator color.

Expected result
Thymol blue colors a solution depending on its acidity.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
What is pH?
Aqueous solutions consist of more than just water H2O If a solution has an excess
of hydroxide ions OH– Combined, H+ Lastly, if a solution has an
excess of H+ |
 |
How do different substances affect pH?


. The latter decreases the solution pH.

appear in the solution, and its pH increases.
Which substances have which pH?
We encounter many substances with very different pH values in everyday life.

Drain cleaners have a very high pH value. A strong alkaline solution makes the organic residue of household waste unstable and easier to wash away.
Carbonates (similar to NaHCO3 and Na2CO3 in the experiment) dissolved in oceanic water make its pH a bit higher than 8.
Foods with a low pH usually taste sour. But there are many drinks with a pH much less than 7 that don't seem to be sour at all.
Stomach acid has a very low pH value. Such an acidic medium is exactly what gastric enzymes need to effectively digest the food you consume.
Why does the liquid change color?
To recap: we prepared five disposable plastic cups, each with small amounts of different substances, and then added the solution of thymol blue to each of them. Since each cup contained a different substance, the molecules of thymol blue found themselves in quite diverse neighborhoods and therefore behaved differently in each.
Let us analyze in detail what happened in each cup. We will refer to the thymol blue as “Ind” (Indicator).
Cup #1 (red)
We put 5 drops of sodium hydrogen sulfate NaHSO4 solution in the first cup. When dissolved in water, it dissociates cheerfully into three charged particles (called ions):
NaHSO4 → Na+ + H+ + SO42-
As a result, many H+ particles (hydrogen ions or protons) are released into the solution in the cup. Due to the large quantity of protons H+, the acidity of the medium increases and the thymol blue (Ind2- – blue) becomes red H2Ind.
Cup #2 (orange)
The citric acid C6H8O7 from the second cup also dissociates in water, producing protons H+. Moreover, each of its molecules can form three whole H+! But, unlike NaHSO4, citric acid does so unwillingly, and as a result, much less H+ is released into the solution in the second cup than in the first. Therefore, some of the thymol blue becomes red H2Ind, and some becomes yellow HInd-. A mixture of yellow and red, just as when we mix paint colors, yields orange.
Cup #3 (yellow)
In the third cup, the mixture of citric acid C6H8O7 and sodium carbonate Na2CO3 produces even fewer protons H+. Therefore, all the thymol blue in such an environment turns into yellow HInd-. In fact, the medium in the third cup is almost neutral. You might think that we would obtain the same result if we just added the thymol blue to a cup of plain water. However, even clear drinking water contains some dissolved carbon dioxide, which makes its medium slightly acidic. That’s why we use a mixture of citric acid and sodium carbonate. Incidentally, when these two substances are mixed, carbon dioxide forms, causing the mixture to bubble.
Cup #4 (green)
Sodium hydrogen carbonate NaHCO3 dissolves in water, dissociating into Na+, H+ and CO32-. Its solution is slightly basic. Therefore, we can observe a slightly green-colored solution resulting from a mixture of yellow HInd- and blue Ind2-.
Cup #5 (blue)
In the last cup, thymol blue is present in its natural form Ind2- and is, as its name suggests, blue – indicating that the medium in the cup is basic. This is the result of water’s interaction with sodium carbonate Na2CO3, which yields the OH- ions responsible for the basic medium:
Na2CO3 + H2O → 2Na+ + HCO3- + OH-
 and hydroxide OH–
and hydroxide OH–