Iodine
Grow crystals out of thin air!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay and in a well-ventilated area.
- Avoid inhaling iodine vapors from the beaker.
- Do not touch iodine crystals even with gloves.
- Remove protective gloves before lighting the splint and put them back on after blowing out the candle.
- Keep a bowl of water nearby when working with fire.
- Keep flammable materials and hair away from flame.
- Do not touch the stove after the experiment. Wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
The air always contains a certain amount of water vapor. Like iodine, it bumps into the surface of the cold cup and transitions into another state. But unlike iodine, it becomes liquid, not solid. We don’t want water to interfere with the growth of the iodine crystals, so we need to remove it somehow. This is where calcium chloride CaCl2 comes in handy! CaCl2 is a hygroscopic substance – this means that it readily absorbs moisture from its surroundings. This curious property of CaCl2 allows us to remove condensation as it appears and observe our shiny iodine crystals.
Step-by-step instructions
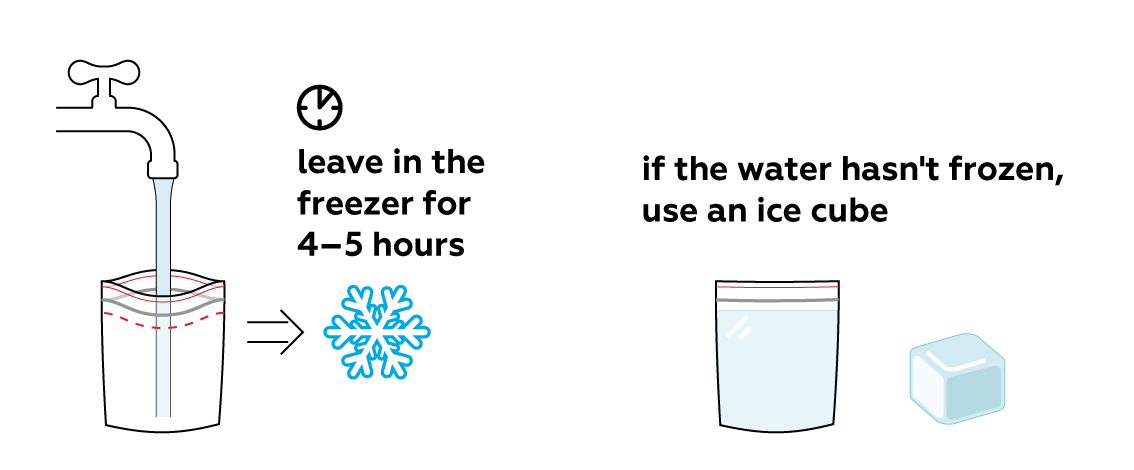
Prepare some ice in advance.
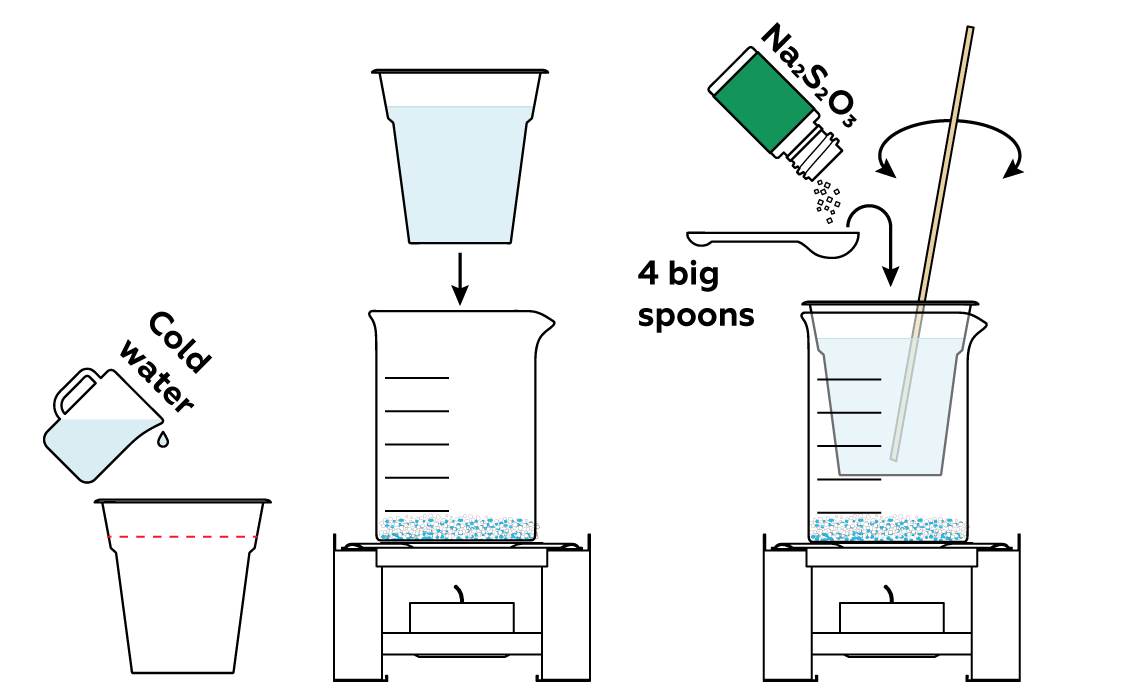
When mixed, copper sulfate CuSO4 and potassium iodide KI react with each other: I- ions share their electrons with Cu2+ and pair up, turning into I2 molecules.

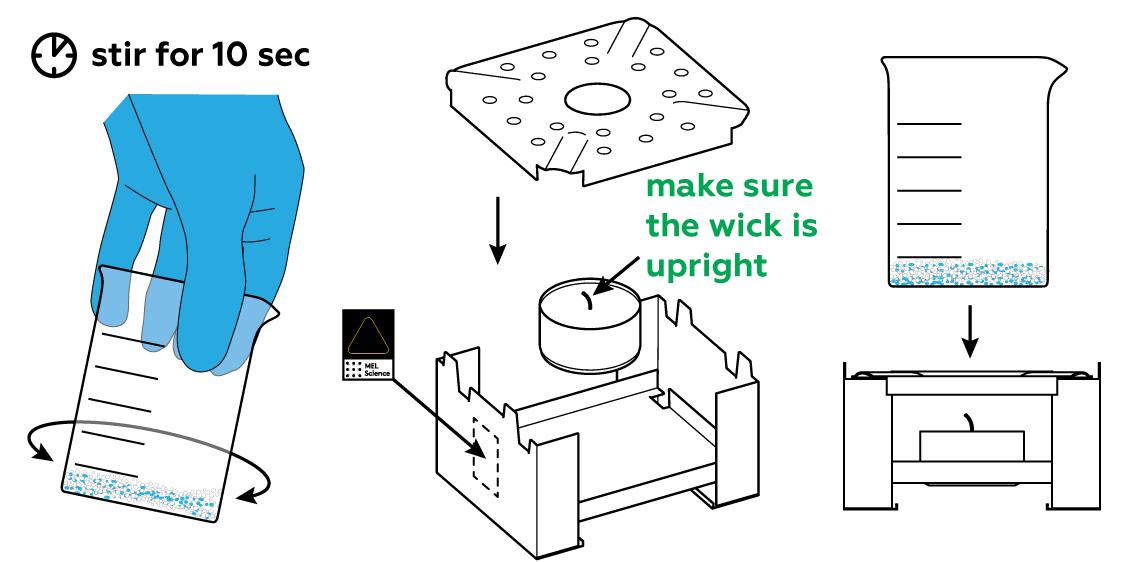
Heat will make the reaction more intense. Stir the mixture and prepare the stove.
Now, add sodium thiosulfate Na2S2O3 to some water—it will come in handy at the very end of the experiment.
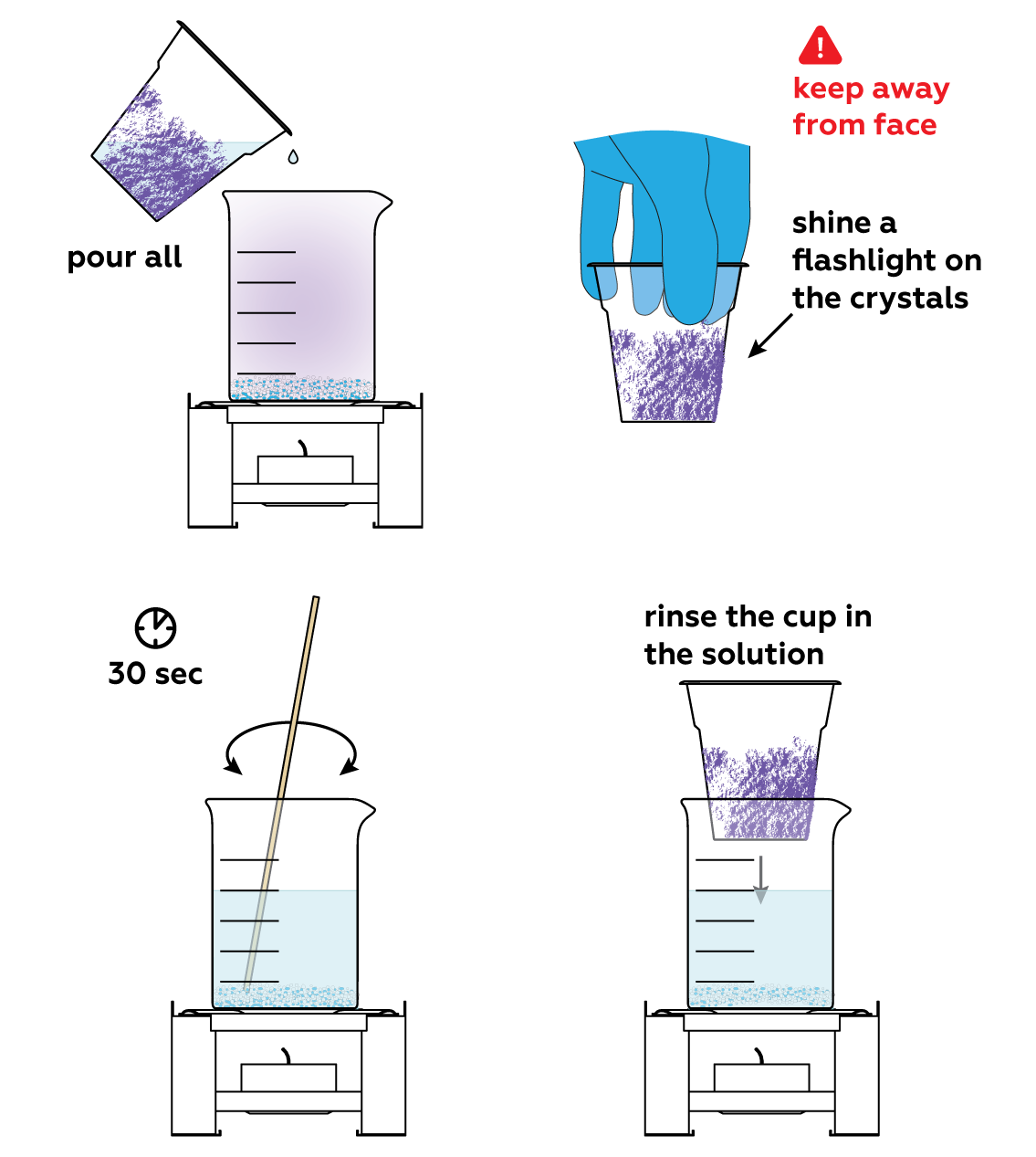
When heated, the mixture reacts intensely, yielding purple iodine I2 vapor. But as the vapor hits the cold cup, it turns into shiny, needlelike crystals.

Do you remember the sodium thiosulfate Na2S2O3 solution you made? This is where it comes into play! Iodine I2 is very volatile and should not be inhaled. But it can easily be turned back into I- by exposing it to Na2S2O3 solution.
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water. Make sure to dissolve all the iodine crystals in the sodium thiosulfate solution.
Scientific description

and shiny crystals
are two states of one substance—iodine
. The transition directly from a gaseous
to a solid
state, bypassing the liquid state, is called deposition. In their gaseous state
, molecules of iodine I2
move quickly and freely, but as soon as they bump into a cold surface
, they lose energy, instantly slowing down and packing closely together to form a solid
. This resembles the process by which snowflakes or frost patterns form from water vapor in the air. However, we usually don't see water vapor in the humid winter air: it is colorless, in contrast to the bright iodine vapor
.

What chemical reaction occurs between KI and CuSO4?
Potassium iodide KI contains iodide ions I− , which are basically iodine atoms
with an extra electron

, a copper atom
with two electrons missing. Cu2+ can take an electron from I−, turning it into I{0}
. The latter
likes to bond in pairs, forming stable iodine molecules I2
. This electron exchange and I2 formation can be written as the following equations:

If we combine these equations, we get this one:
2I− + 2Cu2+ → 2Cu+ + I2
Don’t forget that we also have potassium K+ and sulfate SO42\- ions! They don't change during the reaction, so we can just add them to both sides of the equation in equal quantities. This introduces the formulas KI and CuSO4 on the left. Now the whole equation of the chemical reaction looks like this:
4KI + 2CuSO4 → I2 + 2CuI + 2K2SO4
That’s interesting!
Why is table salt iodized?
Iodine is essential to the production of thyroid hormones, which control many functions like growth and development. The amount of iodine needed for the healthy development of the human body is minuscule. You need only about 1/100000 – 2/100000 of a gram a day! Unfortunately, this element is scarce enough that it can be tricky to obtain even that much from a normal diet. And if a person doesn't get enough iodine, they can suffer slower physical and mental development. Iodine deficiency can even cause a nasty condition known as goiter.
Seafood and dairy products naturally contain sufficient amounts of iodine, but not everyone's diet has enough of these products. Food scientists came up with a brilliant solution: put a miniscule amount of iodine in a food item almost everyone consumes – table salt! Since the introduction of iodized salt in the US, goiter has practically been eliminated, and scientists estimate the average IQ of the US population has risen by 3.5 points due to this measure alone.
Molecular iodine is not used to make iodized table salt. Iodine compounds are used instead. These include sodium iodide NaI, potassium iodate KIO3, and potassium iodide KI, which is what you used in the experiment.