Starch penguin
Make your own starch plastic!
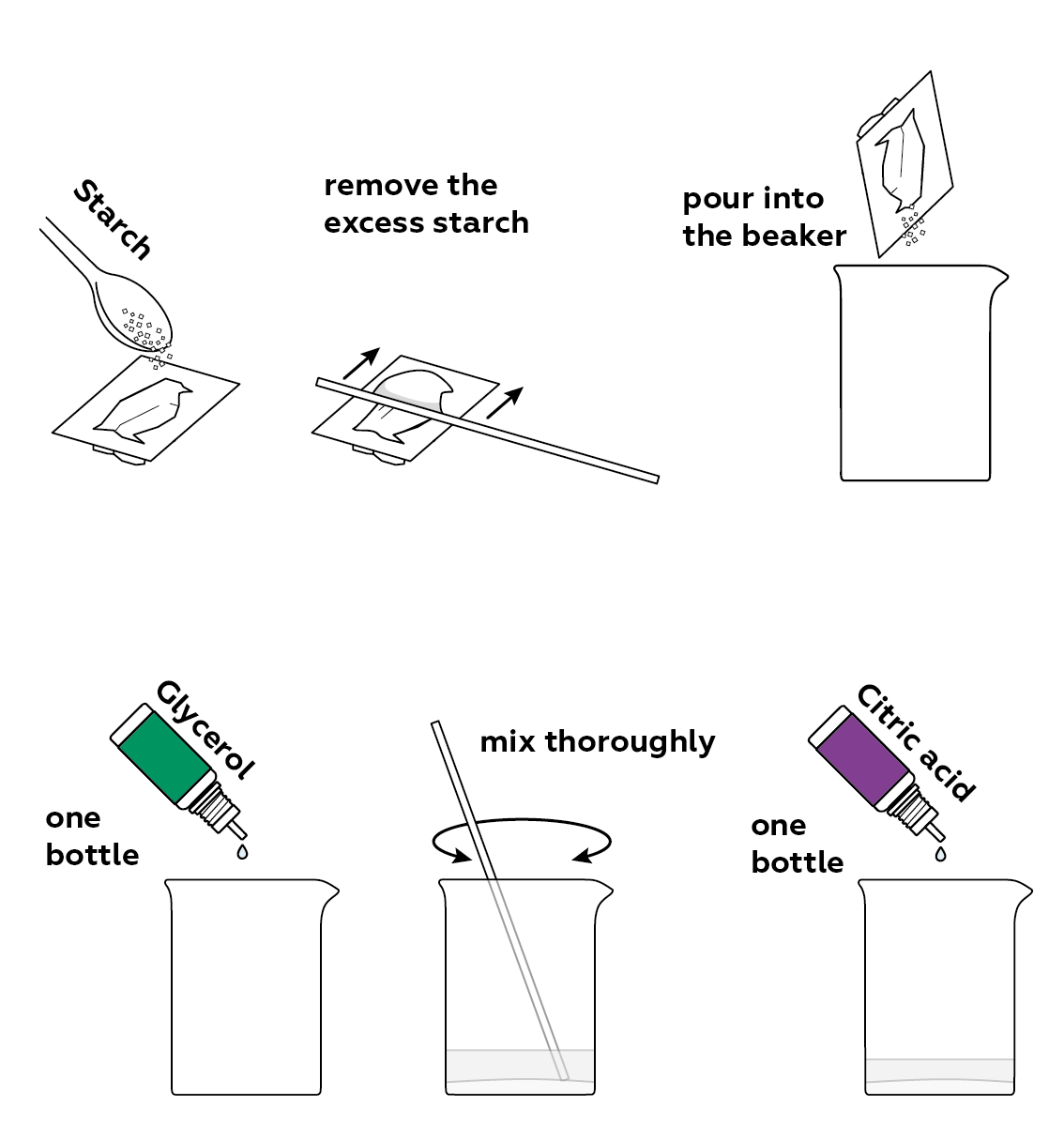
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Remove protective gloves before lighting the splint (step 2).
- Keep a bowl of water nearby while working with fire.
- Keep flammable materials and hair away from flame.
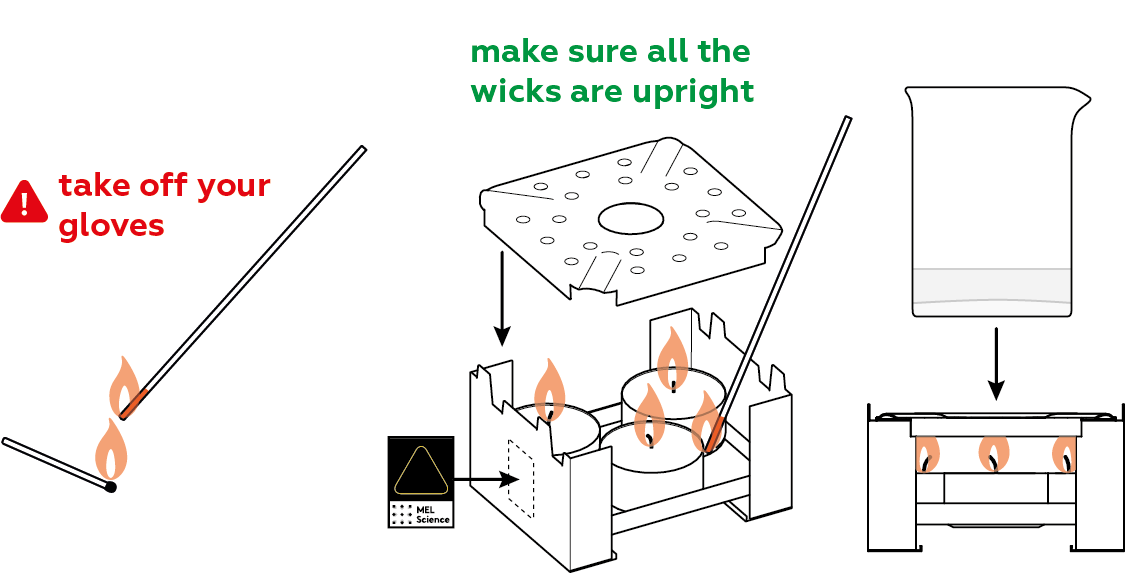
- Place the burner on the cork hot pot stand. Do not touch the burner after the experiment — wait until it cools down.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
If the mixture has thickened, that means you’re doing everything correctly! Continue stirring it and keep the beaker on the stove. The mixture should thin again in about 15–20 minutes.
Use a towel or thick cloth to hold the beaker steady with one hand. This should make it easier to stir with your other hand.
Stir it every few minutes. Try to stir out the lumps to make the structure as uniform as possible.
In some cases, such as if your work area is drafty, it’s possible the mixture didn’t warm up enough when you heated it on the stove. This will make it take longer to solidify, and may prevent it from solidifying completely no matter how much time passes. It’s best to try the experiment again, making sure the room isn’t drafty and the mixture heats up evenly and effectively.
Don’t worry! First, try reheating the contents of the beaker. As soon as the mixture is liquidy again, immediately pour it into the mold. Starch solidifies as it cools.
If this doesn’t work, repeat the experiment and heat the mixture for a shorter amount of time.
Of course! If you extract the starch first.
Take a raw potato (medium-sized should be fine) and grate it into a bowl. Add 150 mL of water and stir for 5 minutes.
Cover your beaker with two layers of cheesecloth and carefully strain the liquid into the beaker. Squeeze the cheesecloth and set the grated potatoes aside. You only need the potato water to obtain the starch.
Allow the starch to settle for about 20 minutes. With time, the starch will settle at the bottom of the beaker. The water may acquire a brown tint.
Carefully pour out the excess water. Most of the starch will have precipitated to the bottom of the beaker. Still, avoid agitating the water as much as possible.
Now you can make a penguin from the starch you obtained!
First, check out the FAQ to learn how to extract your own starch from a potato! You’ll also need citric acid, which you probably already have in your kitchen, and over-the-counter glycerol.
After obtaining your starch, measure about 1 g (half a teaspoon) of citric acid and 4–5 mL of glycerol into the beaker. Add 20 mL of water and a couple drops of thymol blue or any food coloring. And there’s your mixture! Put the beaker on the flame diffuser and continue the experiment according to the instructions.
This is a chemical experiment, so it should be conducted using the relevant protective equipment.
You can safely play with the penguin when it’s dry. Please note: if you used methyl orange in your experiment, avoid touching the penguin without gloves.
Yes. But over time, it will dry out and shrink. After a while, it may even crack. But you can always repeat the experiment using ingredients you probably already have in your kitchen!
Everything’s okay! This steam is water from the heated mixture, and the slight smell is due to reaction products captured in the steam.
This can happen if you leave the beaker on the stove for more than 25 minutes and too much water evaporates. If this happens, we recommend restarting the experiment from the beginning.
If you can’t see any big lumps in the mixture, and if it flows easily and is easy to stir, then you can pour it into the plastic mold.
You can clean the beaker by letting it soak in hot or boiling water for 15 minutes. After that, any remaining traces of the mixture should be easy to wash away. Now you can use the beaker for your other experiments!
Step-by-step instructions
The three main components you need to make starch plastic are glycerol, some acid, and the starch itself.
Now you basically just need to heat the mixture.
At first, the mixture becomes extremely viscous. That's because starch completely dissolves in water, and it's very good at making viscous solutions.
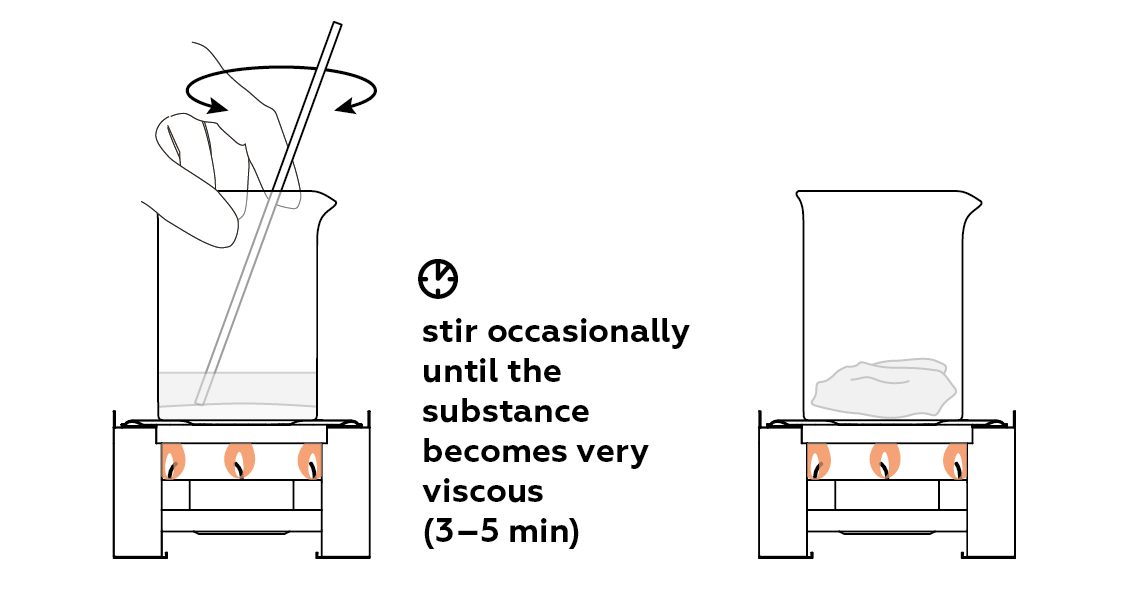
When the mixture turns liquidy again, you can pour it into the mold and let it cool and set.

Enjoy your rubbery-yet-solid figurine made of starch plastic!
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description








What is starch?
Starch is a naturally-occurring compound widely used in the food, farm, technical, and pharmaceutical industries. The most common sources of starch are potatoes and corn, but it is also produced from rice, wheat, and sorghum.
Starch belongs to the carbohydrate family. Carbohydrates are vital to human life; we wouldn’t survive without them. You might already be familiar with such carbohydrates as sucrose, glucose, and fructose, which make fruits taste sweet. Additionally, regular white granulated sugar is actually comprised of sucrose crystals. Starch, however, being a polymer, differs from these sugars somewhat.
Polymers are chemical compounds comprised of very long chains of atoms. These chains usually consist of small, strongly-bonded units called monomers.
Such a comparison is no coincidence: indeed, a polymer molecule closely resembles a chain made up of numerous links. Polymer molecules tend to be quite flexible, and in a solution, they can tangle into odd structures and knots.
While many carbohydrates taste sweet, starch by itself is quite bland. Even so, if you keep a piece of unsweetened starchy food (such as bread or potato) in your mouth for some time, you will begin to notice a faint sweetness. This happens as saliva starts to break down the long chains of starch into smaller molecules, which are essentially sugars.
What happens to the starch?
As we just discovered, molecules of starch can partially decompose when exposed to saliva. The bonds between the links in the starch can also be broken down via exposure to an aqueous acidic solution.
That’s exactly what we do in this experiment. Initially, starch is structurally quite complex: its numerous long chains are tangled with each other, and in some places, these tangles are “stitched” or connected together via “bridge” connections between the main chains. Acid breaks these bonds down, even breaking down some of the long chains themselves.
As a result, the structure of the starch is somewhat modified, and it morphs from a solid compound into a moldable mass.
Why does starch first aggregate into a lump, and only then turn into a thick liquid?
The acid’s effect on starch has a gradual progression. At first, when heated, small particles of starch aggregate into one big lump. The mass becomes quite dense and difficult to stir. However, the acid gradually breaks down the bonds linking the starch particles, and the lump turns into a thick and viscous mass, slowly becoming quite fluid.
Why does the liquid solidify into a pliable mass?
This happens for two reasons:
First, with time, the liquid cools down and the molecules of starch become less mobile. They settle tightly together into closely-packed structures.
Second, the material begins to dry. As fewer and fewer molecules of water are left between the starch molecules, the starch begins to harden and thicken, slowly transitioning from a liquid to a pliable material.
Follow up
A white penguin is nice – but what if you want to add some color? If so, there are a few ways you can do this. For one, when you repeat the experiment, you can add a coloring during step 1. You can use a little bit of food coloring if you plan on keeping your penguin. To obtain a really cool, vibrant orange, you can add a few drops of the methyl orange You can also quickly dip your penguin into the iodine solution you make for another project in this set and see what happens! Don't forget to wear protective gloves. |
That’s interesting!
Why is the penguin rather squishy?
Actually, it’s all thanks to glycerol. The glycerol stops the hardening or thickening process. Otherwise, the mixture would have continued to thicken, and our material would have become as solid and fragile as a crumbly cookie. Once dry, the figure would simply have fallen to pieces.
In order to prevent this, we needed to partially separate the starch molecules from one another. Water cannot serve this purpose because it easily evaporates, but glycerol can. Although glycerol is heavier than water, its molecules are just as comfortable among starch molecules as water is. Moreover, glycerol does not evaporate as easily. Therefore, glycerol acts to guard the plastic consistency of the material, and it can be called a plasticizer.