Periodic table
This is the first lesson where you will meet the periodic table. You will see how atoms are structured in the periodic table depending on their electron orbital configuration.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

At present, scientists have discovered more than one hundred chemical elements.

All the elements are arranged in the modern periodic table in the order of their atomic number.

What is the atomic number of an element? It's the number of protons inside a nucleus.

There is a relationship between the element's electron configuration and its placement in the periodic table. Let's see how it works.

Each atom has a certain number of protons in the nucleus and an equal number of electrons.
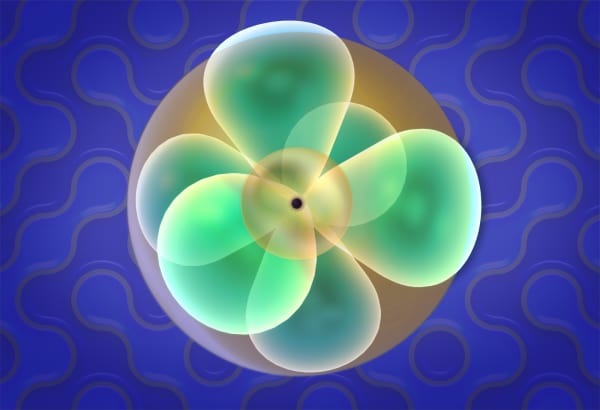
Electrons in an atom occupy orbitals that are arranged in levels and sub-levels according to the diagram on the right.

Let's distribute the atoms according to their electron configurations. First, we copy this diagram.

Then flip it.

As you know, each orbital may contain up to two electrons. So, we divide each square in two.

Now we will arrange our atoms by a number of electrons. We start with hydrogen — the smallest atom, which consists of one proton and one electron on the 1s orbital.

The next is helium. Two protons in the nucleus and two electrons on the 1s orbital.

Now the first row is filled, go to the second one. There come elements with electrons on the 2s orbital.

…and on the 2p orbitals.

The next row contains elements whose electrons fill the 3s orbital.

…and the 3p orbitals.

Look carefully, the next row begins with elements whose electrons occupy the 4s orbital, but then there are elements whose electrons fill the 3d-orbital.

Next, the elements with electrons on the 4p orbital.

The following rows are filled in the same way.

Now you see that we have groups of elements in colored squares: red, blue and green.

Remember that we use color to display what orbital the last electron in the atom occupies - s, p or d.
Let's group the elements under the colors in each row. Look at the first period (the rows in the periodic table are called periods).

Did you see helium? It has a completed electron shell. That is why it is a noble gas that does not react with other atoms. Like argon and neon.
So it makes sense to put it in the group of noble gases.

Remember, the elements are arranged in the modern periodic table in the order of their atomic number - the number of protons in the nucleus.
But the place of the atom gives you information about the electron configuration of this atom.
Teacher's notes
Keywords
element, atom, atomic number, nucleus, protons, electrons, electron configuration, shell, s-orbital, p-orbital, d-orbital, periods, noble gases
Students will
- Learn that there are 118 different chemical elements discovered at the moment
- Learn what elements are arranged in the Periodic Table according to their atomic number
- Study that the atomic number is the number of protons in an atom’s nucleus, which is equal to the number of electrons in the atom
- Learn that rows in the Periodic Table correspond to an electronic level, as soon as the level is full, new elements appear on the next row (level)
- Find out that the rows of the Periodic Table are called periods
- See that neon is the element with a completed electron shell and is similar to other noble gases
- Learn that the position of the element in the Periodic Table provides the information about the electron configuration of the atom
Common misconceptions
The elements on the periodic table are arranged by increasing atomic mass
Hands-on activities
Before VR
- The aim is to give students an idea that elements in groups have similar chemical and physical properties because they have similar numbers of electrons in their outer shell. Show students how Li, Na and K react with water.
- The aim is to show students how they can categories different objects similar in properties. Ask students to sort a set of bolts and nuts in different sizes (3-4) made out of plastic, stainless steel, and brass (discuss that they can be categorized by size or by material).
History and sources of knowledge
- Early attempts to find out natural laws explaining the properties of elements.
- Dalton and Newlands ideas.
- Mendeleev breakthrough: table arrangement showing periodicity in properties.
- Prediction of new elements.
Topics to discuss
- Good scientific explanation not only describes observations but makes predictions.
- Outer electron shell as a source of periodicity.
- Outer electron shell determines chemical properties.
- Group 0, group 1 and group 7 electron configuration and chemical properties.
Fun facts and quotes
- There were approximately 50 elements at the time when Mendeleev created the Periodic Table. He left some boxes empty, expecting that new elements would be discovered and predicted their properties. And they actually were discovered and the prediction of properties matched perfectly.
- New elements are discovered regularly, all of them unstable.
- The only letter not in the periodic table is the letter J.
- Almost 75% of all elements in the Periodic Table are metals.
- Of all 118 known elements, only two of them are liquid at room temperature: bromine and mercury.
Questions
- What is a period in the periodic table?
- What is a group in the periodic table?
- Why do elements in groups have similar properties?
- What are noble gases?
- How many electrons does nitrogen have?
- How many elements are in the second period and why?