Foil etching
Prepare a solution that attacks aluminum foil!

Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Be aware that the reaction mixture can splash.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Yes, you can use any kitchen foil. We don't advise using cupcake liners, as they are too dense to perform the experiment well.
That’s probably okay – the main thing is that the foil not fall off during the experiment.
Restart the experiment from the beginning. Just be careful pouring the water next time!
Step-by-step instructions
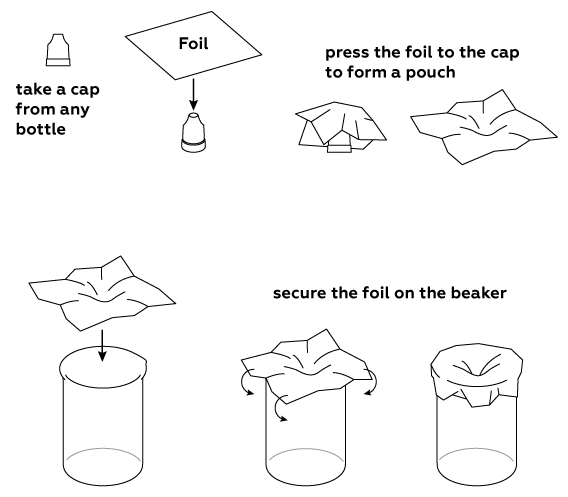
Shape some aluminum foil into a pouch.
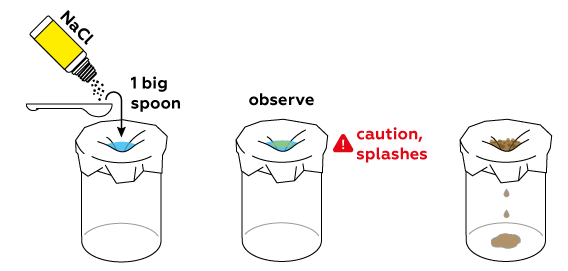
Prepare a copper sulfate CuSO4 solution directly on the foil.

Now add some table salt NaCl and watch.
Disposal
Dispose of solid waste along with household garbage. Pour solutions down the sink. Wash with an excess of water.
Scientific description
Some metals are more active than others, meaning that they are more inclined to abandon their chunk of metal for the solution and engage in other chemical reactions. Chemists have carefully measured this property and assembled a chart known as the "reactivity series." In general, if you take a solution containing ions of a metal 
in it, the ions will switch places—the more reactive metal will go swimming in the solution, while the less reactive one will take its metallic form

As you can see, aluminum Al is much more reactive than copper Cu, but nothing happens when aluminum foil comes into contact with the copper sulfate CuSO4 solution! How come? Unfortunately, it's all a bit more complicated than it first looks. Being quite an active metal, aluminum Al 

When you add some sodium chloride NaCl, a vigorous reaction starts and you can clearly see brown-red copper metal flakes forming—but why? Cl\- ions are able to compromise the otherwise-strong Al2O3 layer 

