Effect of iodine on starch
Helpful reaction

In this article, you will learn how the reaction of starch and iodine takes place. This chemical process is not only very interesting, it is also practical, as it is often useful in everyday life, when we need to find out whether a certain product contains starch.
To start with, let’s define what starch is.
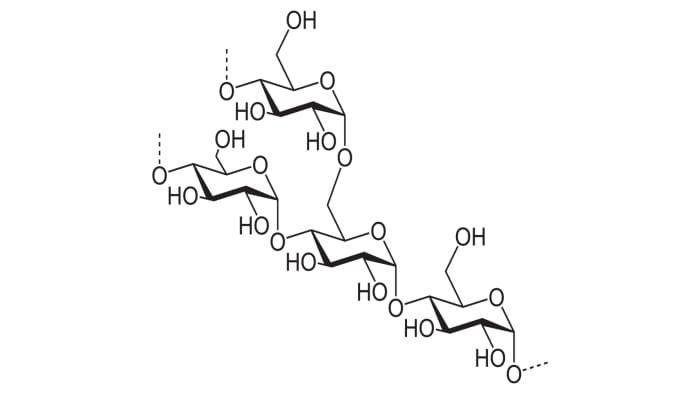
It’s a tasteless, white powder resembling flour in its consistency, and with the formula (C₆H₁₀O₅)n – a polysaccharide consists of amylose and amylopectin.
Starch is the result of a natural process – photosynthesis. For plants, it serves as a supply of nutrients, and for the human body, it serves as a source of important carbohydrates.
Physical properties of starch
Starch is insoluble in cold water. If you press the powder with a spoon, squeezing it, you hear a characteristic creak, caused by the friction of microparticles against one another.
Chemical properties of starch
In hot water (C₆H₁₀O₅)n does not dissolve either, but swells up to a thick and viscous substance, forming a colloidal mixture known as a starch paste. A solution of starch in water is a non-Newtonian fluid (liquid that changes its density and viscosity when exposed to physical force).
If you add acid to water containing starch, for example, H₂SO₄, you can observe the hydrolysis process with a reduction of the molecular mass of the substance and the formation of “soluble” starch.
Starch contains different polysaccharides in its structure.
Starch is also a multiatomic alcohol, which forms ethers and esters during certain reactions – intermolecular dehydration and etherification.
Starch is obtained industrially from wheat, potatoes, corn, and rice.
It is also not difficult to obtain starch in the home.
Application of starch
Starch is widely used for industrial purposes. It can be used to obtain such substances as: glucose, molasses, ethanol.
Starch is also widely used in textile manufacture, and for treating fabrics. In paper factories, the powder is used as a hydrophilic agent, a material that increases durability and improves the typographic qualities of paper. It is also used for manufacturing medicine and food.
In the household, starch is used by practically everyone, as it is used to starch clothing, make jelly, and prepare pastes by mixing starch with water and flour etc.
Starch-iodine reaction
In this article, we will use a 5% alcohol iodine solution which is used in medicine.
Starch interacts with iodine, forming inclusion compounds, which are called clathrates. This chemical reaction was discovered back in 1814 by the scientists Jean Jacques Colin and Henri-François Gaultier de Claubry.
Inclusion compounds are special compounds in which the molecules of one substance enter the molecular structure of another substance.
In this case, amylose molecules (one of the main polysaccharides of starch) will be the “hosts” and the iodine molecules will be the “guests”.
Experiment with starch and iodine at home
This is a quite simple chemical experiment which can be carried out at home and shown to children, to inspire them with a love for chemistry.
You will need:
- glass test tube;
- alcohol iodine solution;
- pinch of starch;
- lukewarm water;
- stirring rod.
Pour water into the test tube and add 4-5 drops of iodine. Add a pinch of starch and mix well with a rod. As a result will be a dark blue solution.
By the way, you can also repeat this experiment in other ways, for example, add one drop of iodine to a small mound of starch, and a dark blue patch will appear. You can also drip iodine on to half a potato, as it has a high starch content. If you immerse a peeled potato in cold water, starch particles will appear in the water after a certain period of time. If you hold a peeled potato in your hands, they will also become coated with starch.
If you heat a test tube containing a solution of starch, iodine, and water over a chemical burner for some time, the solution will turn white and transparent. This is because the compound of iodine and starch is unstable, but if you put the test tube in cold water, a dark blue sediment will form once more.
When starch is heated to the boiling point, it begins to break down, and the chains of amyloses break, thus forming short chains of dextrins, so the color starts to change. Glucose does not give any color in a reaction with iodine.
An interesting fact: amylopectin (another polysaccharide of starch) gives a purple-red coloring when reacted with iodine. There is significantly more amylopectin in starch than amylose, which gives a blue color, but the blue color overrides the red-purple color.

Let’s see how the reaction of starch and iodine can be useful in daily life.
It’s simple: if you have two unlabeled containers of soda and starch at home, and you don’t want to find out which one is which by tasting them, add a drop of iodine.
Some food products are forged with starch, because of its viscous structure. This especially concerns honey: you can often find fake honey for sale at markets containing a large amount of (C₆H₁₀O₅)n. Once again, you can detect starch using the same simple chemical method with absolutely any food products.