Molecules
During a journey inside different materials (water, sugar, etc.) you will see that the atoms in them arranged in bigger groups called molecules.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript
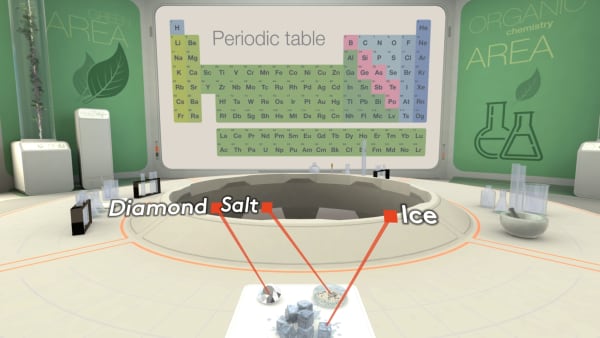
Today in our lab we have diamond, salt, and an ice cube.
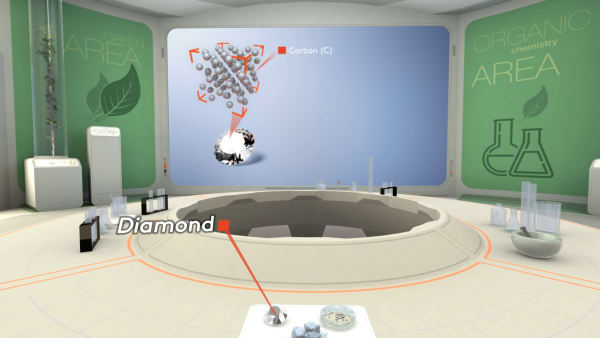
Remember that a diamond consists of carbon atoms. Each atom is strongly bonded to four others and the atoms are arranged in a specific order.
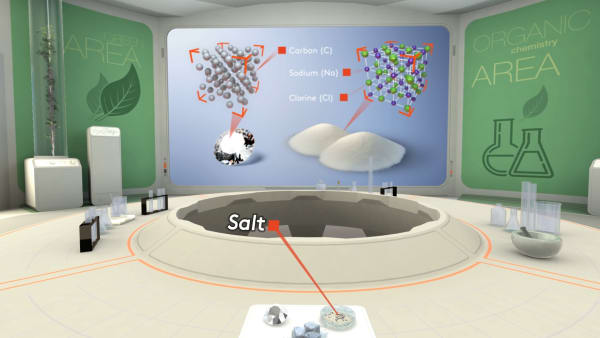
Salt is an ionic substance. In salt, sodium ions and chloride ions are packed in a regular array.
But what will we see inside ice?
Let's look inside.
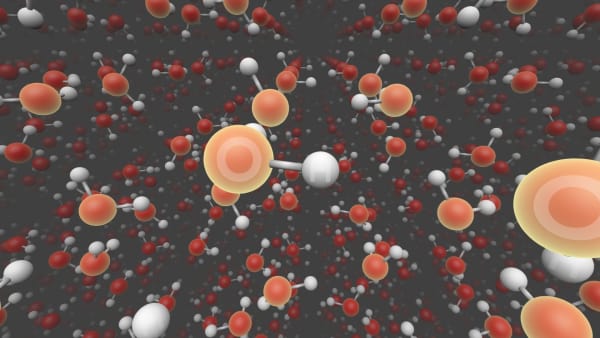
We see two types of atoms here: oxygen and hydrogen.
They are grouped in groups of three atoms.
Inside one group atoms are bonded to each other.
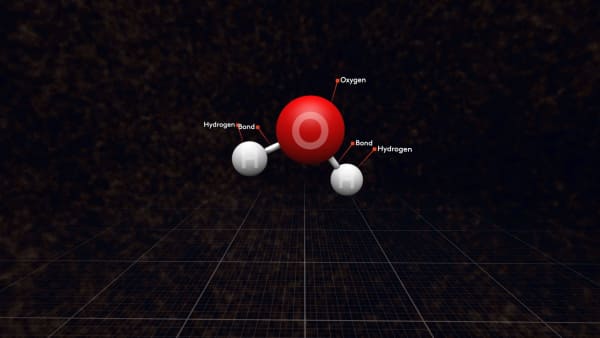
These groups are called molecules; water molecules in this case.
Each water molecule contains one oxygen and two hydrogen atoms connected by strong bonds. We show them by sticks between atoms.
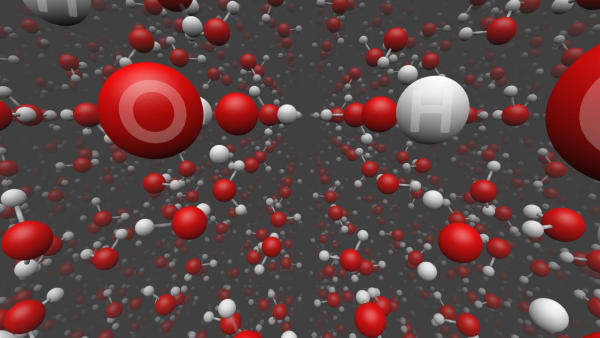
Connections between molecules are many times weaker and are easily broken by heating.
Let's go to the lab and look at the melting ice.

So when you pick up ice in your warm hand, the connections between molecules are broken and it turns into liquid water.
What do you think, do water and ice consist of the same molecules or not?
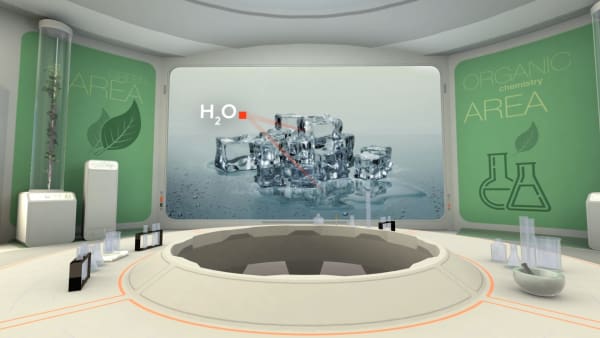
Yes, they do. Heat from your hands is not enough to break water molecules.
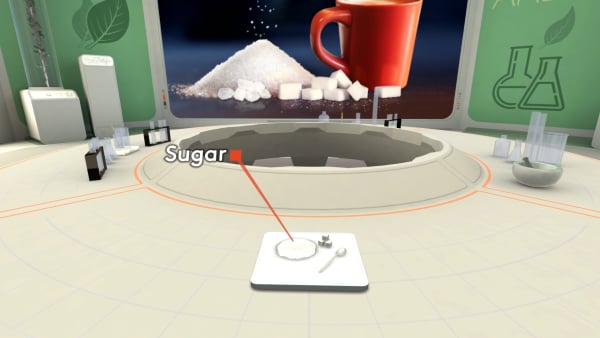
Now check out something else.
On the table, you see the ordinary sugar.
Let's look at its molecules.
Do you see the sucrose molecules?
These molecules are much bigger than water molecules.
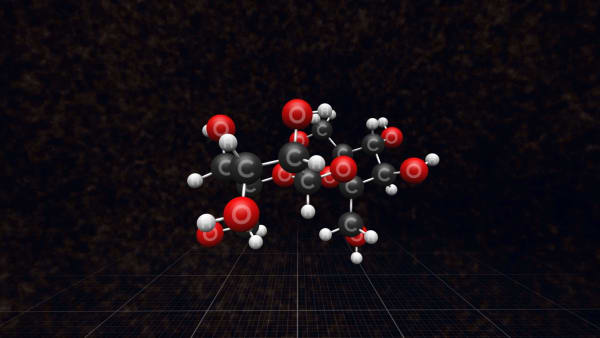
Look at one of them.
Each molecule contains 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms.
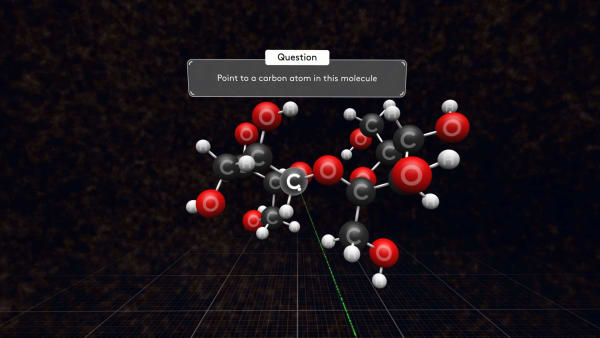
Point to a carbon atom in this molecule.
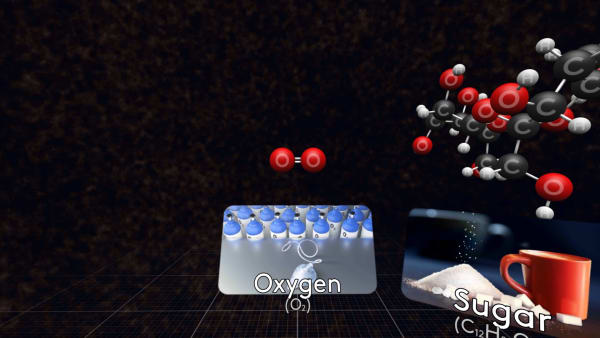
Let's look at some other molecules of substances you know.
Some molecules are small, like this oxygen molecule, that consists of just two oxygen atoms.
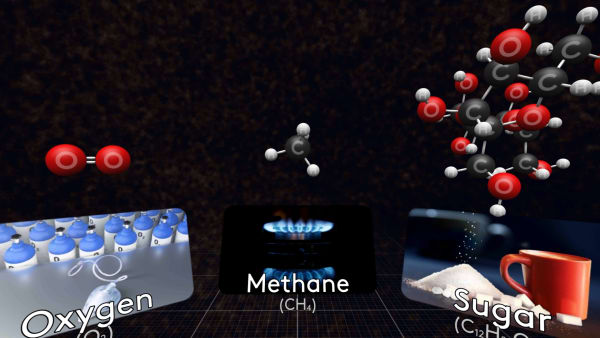
This is a molecule of methane, the main component of natural gas.
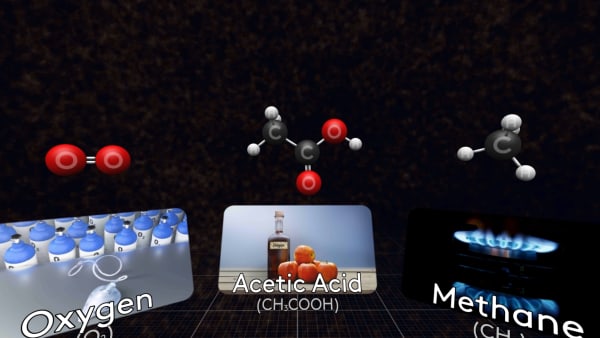
And here is a molecule of acetic acid. The solution of this acid in water is called vinegar.
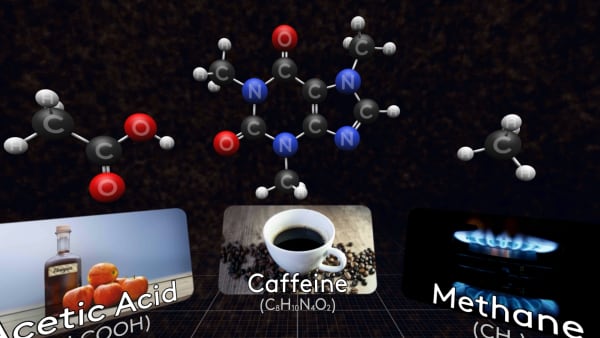
Some molecules are much bigger, like this caffeine molecule found in coffee, tea, and cola.
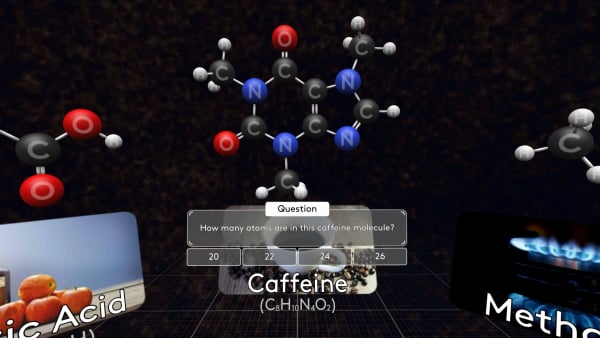
Try to count how many atoms are in this caffeine molecule?
You saw that, in some substances, atoms are grouped into molecules.
These substances are called molecular substances.

Let's go to the lab again.
The smallest molecule in the world is a hydrogen molecule. It consists of two hydrogen atoms.
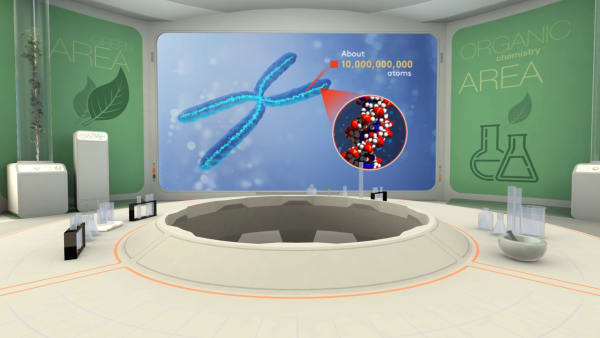
One of the biggest molecules in the world is DNA, it can contain hundreds of billions of atoms.

There are as many atoms in one molecule of DNA as there are stars in a typical galaxy.
Teacher's notes
Keywords
molecules, atoms, bonds
Students will
- Learn that nearly all substances consist of molecules
- Find out that bonds between molecule atoms are strong and bonds between molecules are weak
- Find out that molecules can be small and large
Hands-on activities
After VR
Ask students to heat two substances (table salt and crystals of an organic compound, such as sugar, benzoic acid, etc.) with a low melting point. After the experiment, ask the students which substance is more probable to be molecular and why.
History and sources of knowledge
- The law of definite proportions was a big step towards the understanding of chemical compound formation.
- Modern methods of analysis like spectroscopy and mass-spectrometry show molecular composition.
Topics to discuss
- Particulate nature of matter (particles can be atoms, ions or molecules).
- The forces that keep atoms in molecules together. The forces that keep molecules together.
Fun facts and quotes
- There are up to 2 trillion molecules in one human cell. More than half of this number are water molecules.
- When you tear off plastic like polyethylene or polypropylene you actually tear off its molecules.
- Nearly all organic compounds are of molecular structure.
- Most smelly substances are of molecular structure.
Questions
- Which are stronger bonds between atoms in a molecule or bonds between molecules?
Calculating
Calculate the number of atoms inside a molecule (hydrogen, water, acetic acid).