Orbital names
Now that students know that electron behavior in atoms is described using orbitals, it is time to learn the orbitals' names. Students will see electrons added to an atom one by one and learn the names of the orbitals.
This lesson is a part of MEL VR Science Simulations. Learn more →
Similar lessons
Transcript

Remember you have seen electron orbitals that have different shapes? Today we will learn their names.
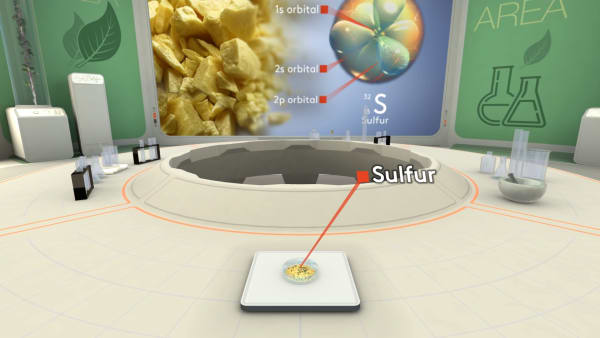
Let's take a piece of sulfur and look inside to see its atoms. Ready to dive?
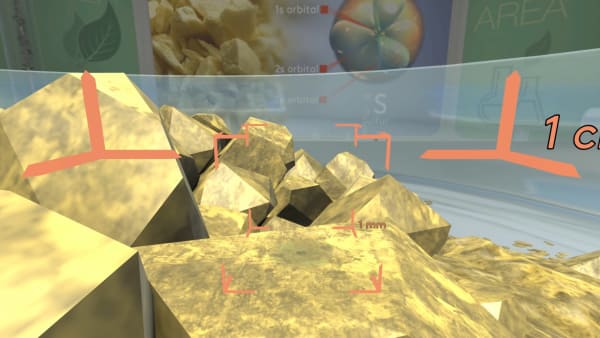
We have to zoom in a billion times to see individual sulfur atoms.
Let's choose one of those atoms and get closer.
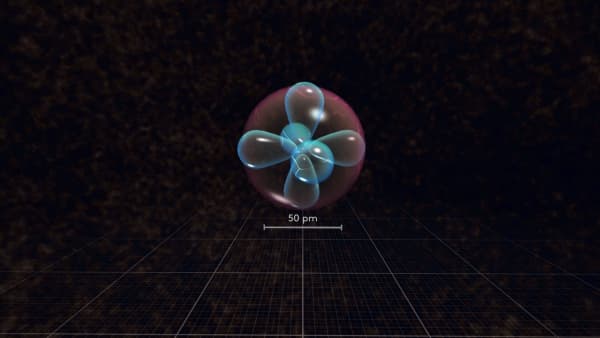
You see a lot of different electron orbitals.
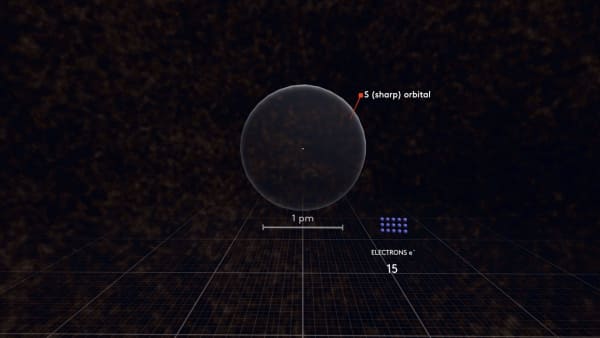
Let's remove all the electrons and add them back one by one.
As you may remember, the first electron orbital has a spherical shape. We call this orbital S. Indeed, we call it 1s to show that it is the first spherical orbital.
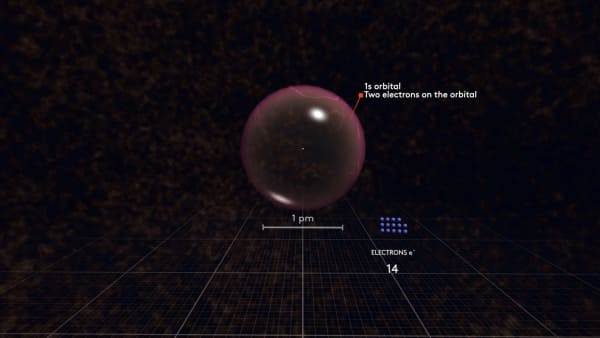
Two electrons can fit in one orbital, so the second electron will go to the same 1s orbital.

The third electron cannot be added to an orbital that already contains two electrons.
So, it will go to the next orbital.
This spherical orbital is called 2s. The next electron will go there as well.
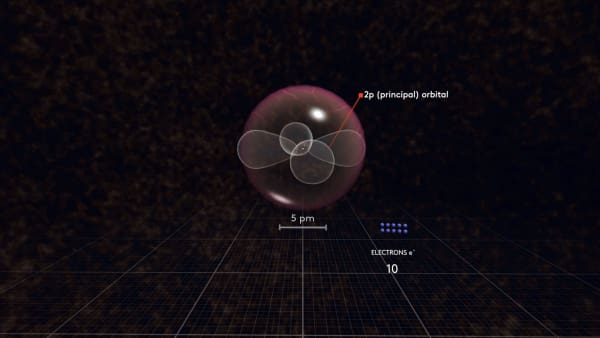
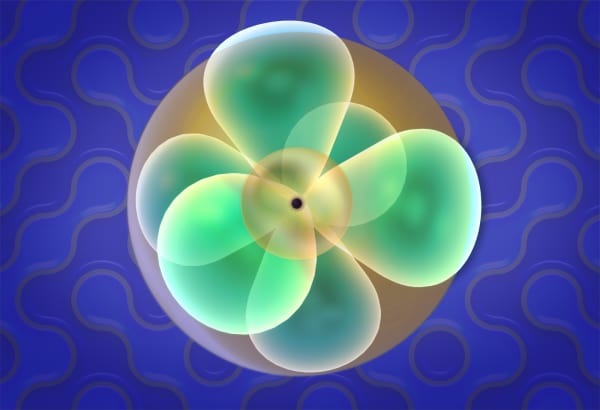
The next orbital is called p or 2p, to indicate it is located in the second shell.
Now watch carefully what happens with the next electron. It will not go to the same orbital but will rather go to another p orbital.
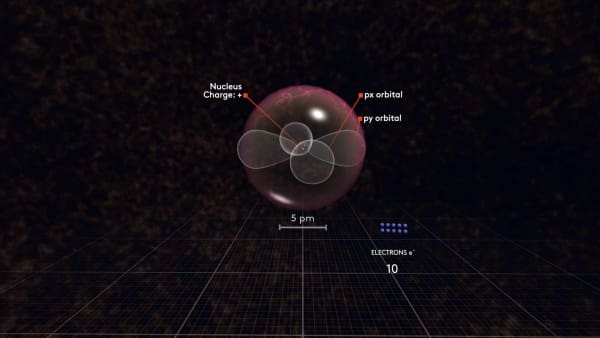
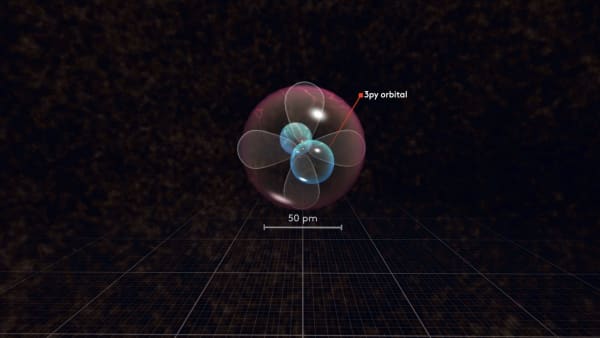
To distinguish between these p orbitals, we will call our first orbital px and our new orbital py. But why did not it go to the same orbital as the previous electron?
As you may remember, electrons are attracted to the positively charged nucleus. So, they want to be as close to the nucleus as possible.
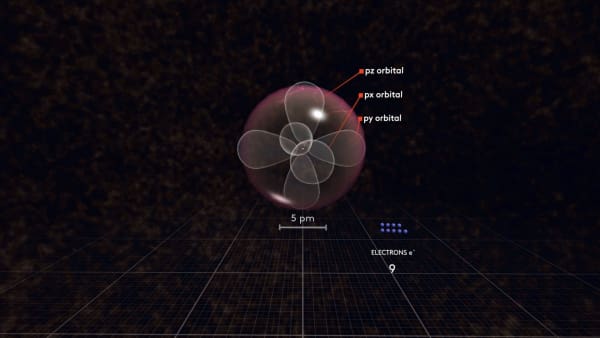
But at the same time, negatively charged electrons repel each other. So, if there are two orbitals the same distance from the nucleus, the electrons prefer to be in different orbitals, farther away from each other.
Following this logic, the next electron will go to the third p orbital called pz. There are only three p orbitals so the next electron must share an orbital. Let's continue adding electrons.
Now all the 2p orbitals are full; there is no space for any additional electrons in them. Therefore the next electron will go farther away from the nucleus to the next spherical orbitals.
Try to guess its name.

It is called 3s.
We will continue adding electrons and show the names of the orbitals.
Now when we have added 16 electrons our sulfur atom is complete.
Let's go back to our laboratory.

Try to recall the maximum number of electrons that can share the same orbital?
Only two electrons can share the same orbital. In the next lessons, you will learn the reasons for that.
Teacher's notes
Keywords
electrons, electron shells, orbitals, s-orbital, px-, py-, pz-orbitals
Students will
- Recall that electrons in atoms are on differently-shaped orbitals
- Learn that one orbital can fit two electrons
- See in what order orbitals are filled with electrons
- Learn orbital names
Hands-on activities
After VR
The aim is to give students a “real life” analogy to the order in which electrons appear.
Students are given a “hotel activity sheet” and are asked to allocate guests in the hotel according to the rules.
Rules for guests:
- No more than 2 guests per room.
- The lift is broken, and the guests are lazy: they will always try to get a place in a room closest to the ground floor.
- Guests will try to avoid neighbors if it doesn't cause them to use the staircase.
History and sources of knowledge
- Quantum theory: description, computational models.
- Atomic spectra with shell series. Zeeman and Stark effects clearly show the number of p-, d-, and f-orbitals.
Topics to discuss
- How can we trust theories about things we can't see?
- Quantum theory
- Rules for electrons: Pauli Exclusion Principle, Aufbau Principle, & Hund's Rule
Fun facts and quotes
Names of the orbitals: s-, p-, d-, and f- can seem like a random set of letters. Early spectroscopists who researched the series in spectra of alkali metals could see different sets of lines for each electron shell. They categorized these lines as sharp, principal, diffuse, and fundamental. The orbitals were named after the corresponding sets of lines.
Questions
- How many electrons can fit into one orbital?
- What is the electron fitting order for a lithium atom?
Quiz
Please see below for the link to a Google form containing a quiz on materials from two lessons: Electron Orbitals and Orbital Names.
This can be assigned during class time or as homework. The quizzes are marked and the system shows which questions students get correct and incorrect. Please note that students should record their scores, as they will not be viewable later.