When green is red
Take the green from green leaves!

Reagents
Safety
- Do not aim UV light at eyes or face.
- Put on protective gloves and eyewear.
- Conduct the experiment on the safety underlay.
- Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
Step-by-step instructions
How can you take the "green" out of a green leaf? First, you need to crush the leaf, because the "green" is contained deep within its structure, behind tough walls.
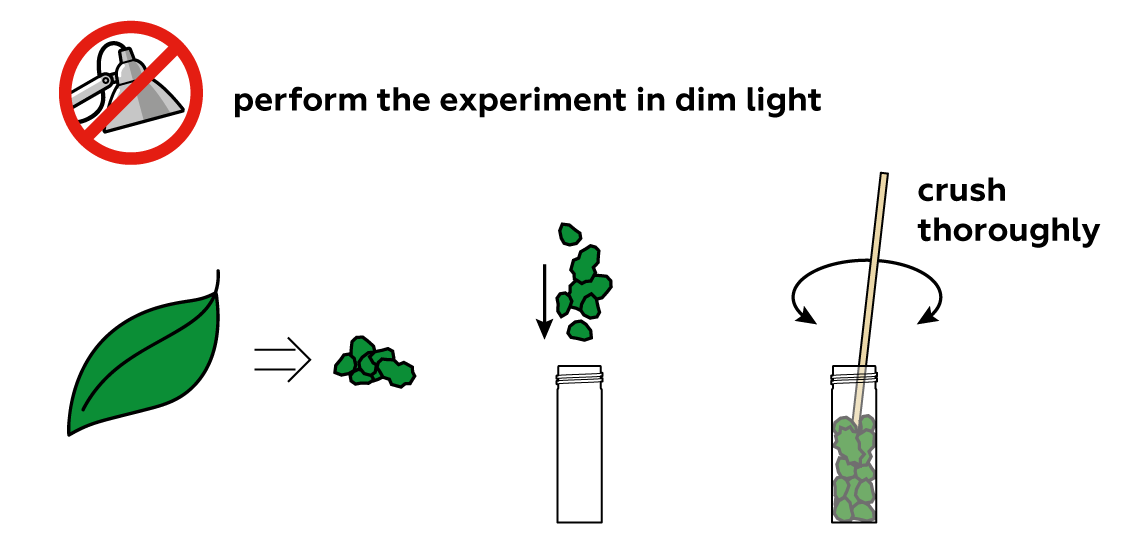
The "green" in plant leaves is actually a set of similar chemical compounds called "chlorophylls." To separate them from the remainders of your leaf, you can just "lure" them into a liquid. Water is often a very good liquid for similar purposes. Chlorophylls, however, don’t approve. They prefer other kinds of liquids, like oils, alcohols, or triethyl citrate.
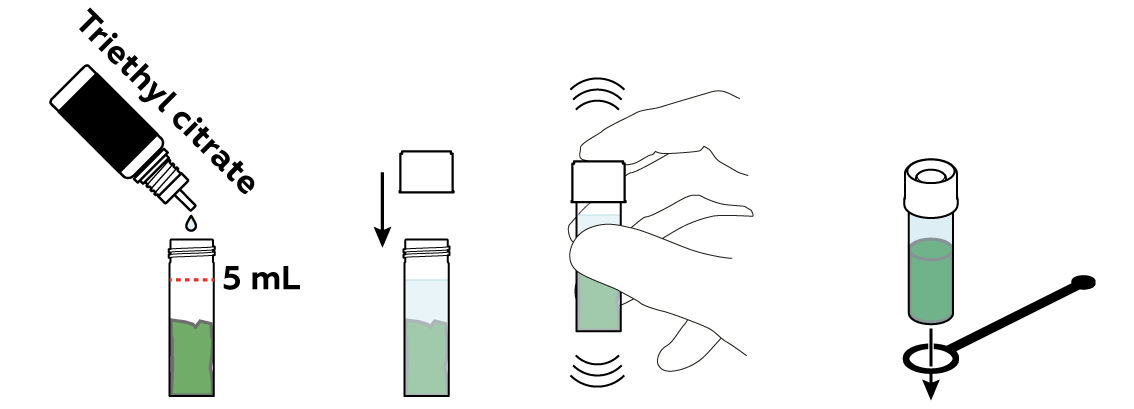
The chlorophylls don’t migrate into triethyl citrate exceptionally fast, but you can speed them up by heating the mixture.
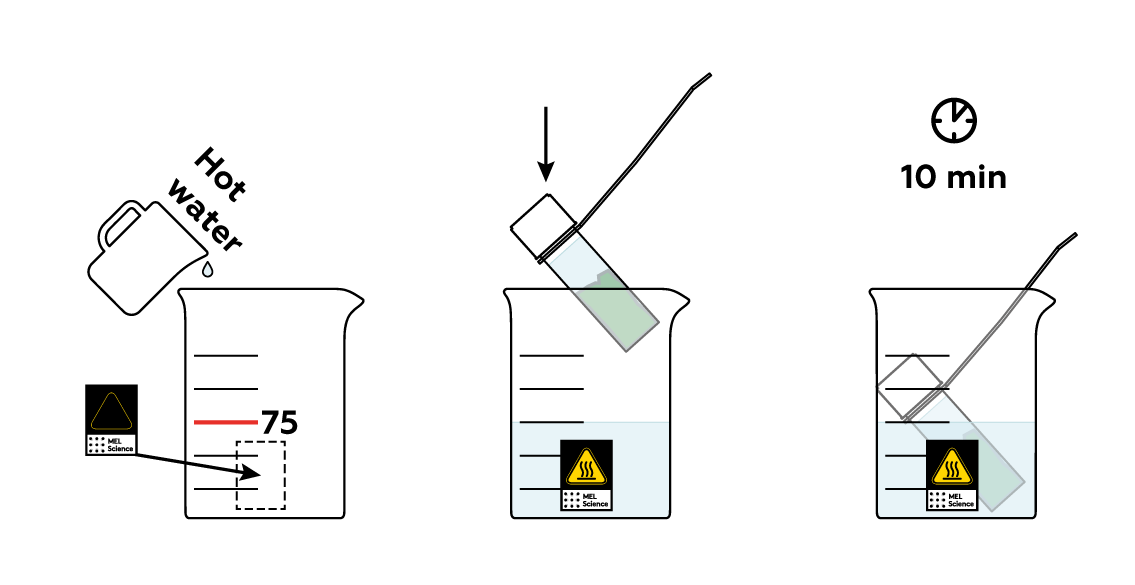
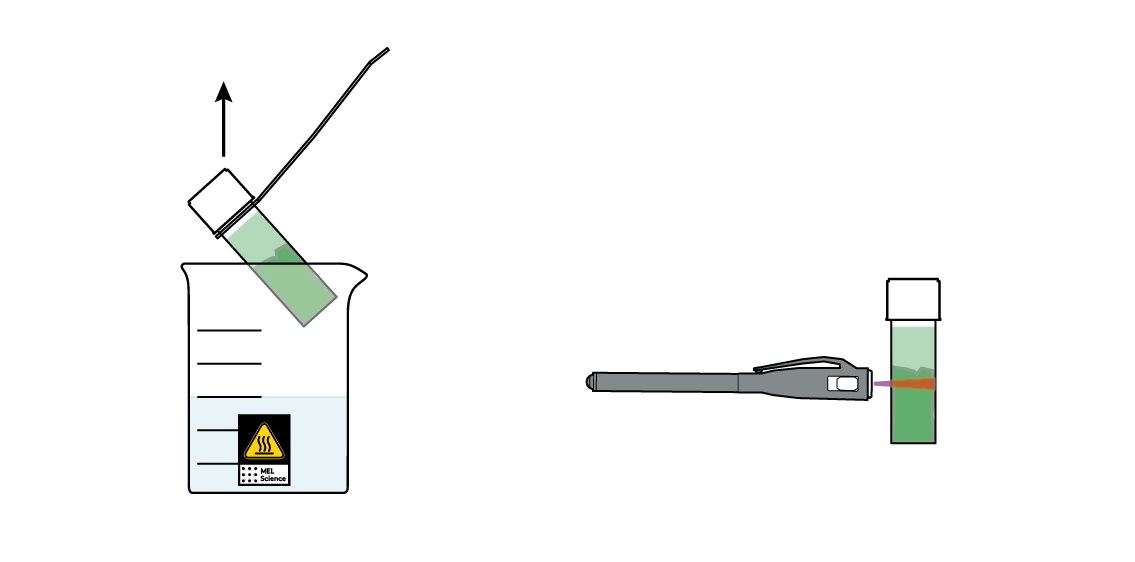
You've got most of your leaf's "green" dissolved in your triethyl citrate! Isn't this liquid pretty green? Or is it? Take your UV light and shine it at your vial. This green is sometimes actually kind of red!
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
Your eyes aren’t deceiving you; chlorophylls are actually green. But why did you see red? When you shine regular white light on chlorophyll, you’re actually illuminating it with all the colors of the rainbow. That's because white light is really a mixture of all these colors. Red, green, blue, yellow, and violet all hit the solution at the same time. However, most of the blues and reds get stuck in it, and only the green portion of the light is able to pass through the liquid or bounce back into your eyes. Hence, you perceive the solution to be green.
The light from the UV flashlight, however, contains no green whatsoever. Conversely, it doesn’t contain any red, either; it consists of barely-visible blue/violet and invisible ultraviolet light. So the solution should look, if anything, violet—why does it look red instead? As it turns out, the light from the flashlight is absorbed by the chlorophyll, but it's not lost. In fact, the ultraviolet light causes tiny changes in the chlorophyll molecules, causing them to get "excited." They immediately change back, however, and in doing so, they release some light back. And this light happens to be red
Chlorophyll isn’t the only plant-based substance that behaves in such a manner. Curcumin, for instance, is also soluble in triethyl citrate and has a beautiful yellow-green glow to it!
That’s interesting!
Why do plants need chlorophyll, anyway?
In general, plants use chlorophyll to utilize solar energy to form glucose C6H12O6. They accomplish this by spending photons' light energy to combine carbon dioxide CO2 and water H2O, releasing oxygen gas O2 as a byproduct:
6CO2 + 6H2O + light energy → C6H12O6 + O2
The energy gained from oxidizing glucose is later used by the plant to produce other organic substances.
Chlorophyll alone doesn't produce glucose. In fact, it only acts as a "solar panel" to catch sunlight and transform it into chemical energy, which is later used by complex cell machinery to produce glucose. Interestingly, plants borrowed the whole sun-powered "glucose factory" concept from bacteria! In fact, plants' ancient ancestors simply incorporated some photosynthesizing bacteria into their cells, and nowadays these bacteria live on in modern plants' cells as specialized organelles called "chloroplasts."
All green plants contain chlorophyll, but not all chlorophyll-containing plants are green. As you know, most plants absorb red and blue light from the spectrum emitted by the Sun, reflecting green. Some plants, however, have found a way to utilize green light too. These plants use other pigments, carotenoids, to turn green light into yellow, orange, or red light. These plants may seem exotic, but they aren't: look around your local grocery store and you may find blue basil or blue cabbage, which contain carotenoids called anthocyanins. The diversity of autumn leaves' colors is also due to carotenoids. When green chlorophyll decays, some leaves turn red, orange, or yellow if they contain carotene, lycopene, lutein, or other carotenoids