Iron gall ink
Make ink from iron sulfate and tannin!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Try repeating the experiment from the very beginning using a new sheet of paper. Make sure to put enough drops of tannin on the brush. Wait 5 minutes for the solution to soak into the paper. Also, make sure you apply a sufficient amount of iron(II) sulfate to the cotton cylinder and coat the sheet of paper with care.
There is enough paper in the set to repeat the experiment several times. If you really want to use ordinary paper, you can certainly try! Document any differences in outcome and let us know the results of your observations!
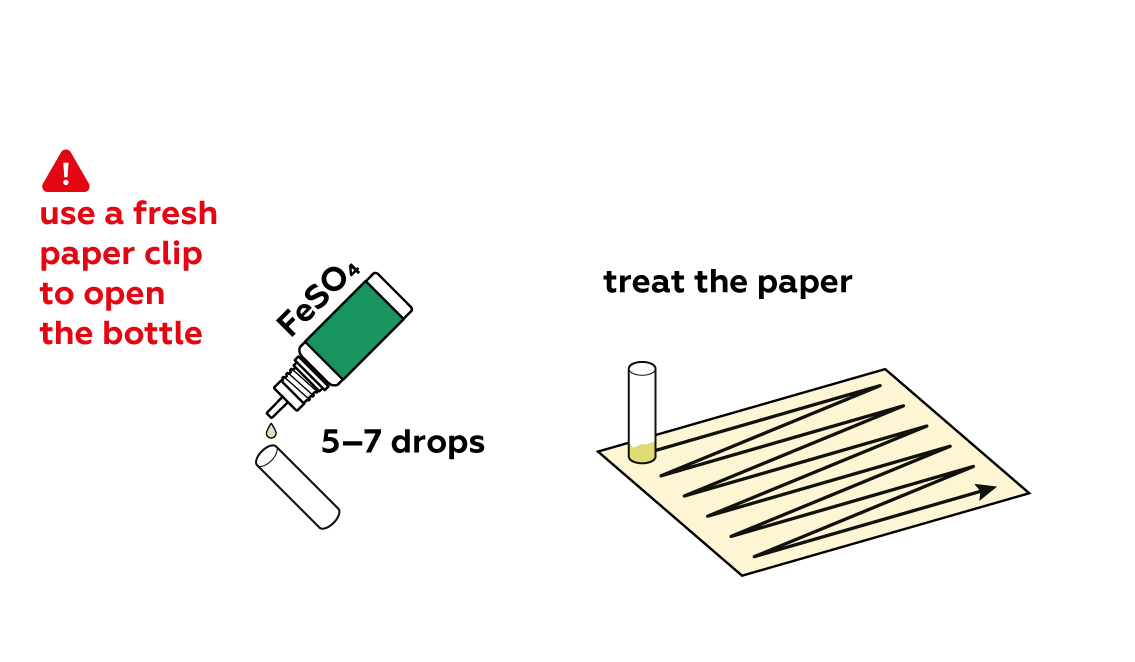
Good question! If a paper clip with some tannin solution on it is used to open a bottle of iron sulfate, the iron sulfate may be contaminated; the substances will react with each other and the experiment may not work as intended.
Step-by-step instructions
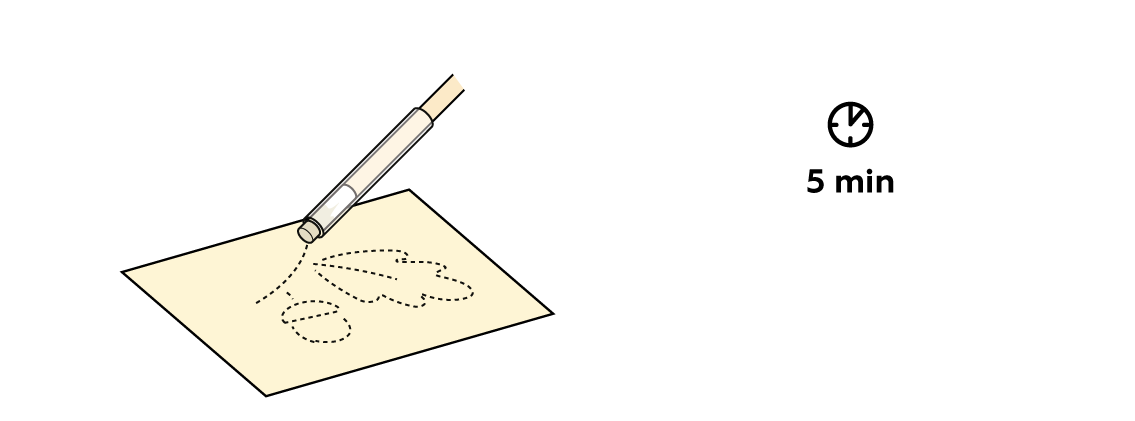
Fill a pen with tannin solution. This compound is non-volatile and rather pale, and thus is well-suited to the role of invisible ink. As long as you know how to make it visible, at least.

Tannin solution doesn't have a very intense color. And a small amount on paper is practically invisible.
Iron sulfate FeSO4 solutions are also relatively pale, but apply some to the paper with your tannin inscription.
The tannin combines with Fe2+ from the FeSO4 to form a strongly-colored compound, allowing you to see the message clearly.

Expected result
The reaction between tannin and iron(II) sulfate gives the text a dark coloring. The secret message becomes visible!
Disposal
Dispose of solid waste together with household garbage. Pour solutions down the sink and wash with an excess of water.
Scientific description


Why does the secret message appear?
Tannin molecules can bind with metal ions such as iron Fe or copper Cu (see the “Follow-up” section to learn how to reveal a secret message written with copper sulfate CuSO4). The reaction forms complex molecules with a bold, dark color. Hence, the interaction of a tannin solution with an iron sulfate FeSO4 solution (containing iron(II) Fe2+ ions) renders the secret message visible.What are tannins?
Tannins are a compound class of plant origin. They can be found in tea leaves, nuts, oak, and some tree bark. Tannins are brownish-yellow and have an astringent taste and a discreet, pleasant aroma. If you’d like, you can try carefully smelling the solution. Do you know the proper way to smell chemical substances? Hold the container in one hand approximately 20–25 cm (9–10 inches) from your face. Use your other hand to gently “fan” or “waft” the air above the bottle towards your face. The main ingredient of the tannin solution in the set is tannic acid, which is one of the most easily-available tannins.
Follow up
Copper sulfate and tannin
In addition to iron(II) Fe2+ ions, tannins can also bind to other metal ions such as copper(II) Cu2+.
Solutions of tannin and copper sulfate CuSO4 are both almost colorless. Try writing a message with CuSO4 solution, then using a tannin-soaked absorbent to reveal your message.
You can also do everything in reverse: write your message with tannin solution and then make it visible using an absorbent wetted in copper sulfate CuSO4 solution.
That’s interesting!
Tannins can be found in many plants, often protecting them from the encroaching outside world. Tannins can bind to protein molecules, thereby inhibiting the activity of various microbes and preventing them from attacking their host plant. Moreover, most animals and insects dislike the astringent taste of tannins and avoid plants where tannins abound.
Because of their ability to bind with proteins, tannins are used as hardening agents for leather and even as a topical anti-inflammatory medicine.
Moreover, tannins’ restorative powers are utilized in the medical industry to treat bleeding, bowel dysfunction, and diarrhea. Tannins can act as an antidote to mercury or lead salt poisoning: they bind firmly to the cations of heavy metals while staying soluble in water, which allows them to pass through and out of the body.
Writing became a tipping point in the growth and development of human civilization. For a long time, people used carbon and various soot-based inks to put their thoughts on paper (or parchment).
Centuries ago, people discovered that mixing certain leaf extracts with iron salts produces permanent inks that are much more water-resistant. These then-revolutionary inks bind so strongly to paper thanks to the fact that their Fe2+ cations are oxidized into Fe3+ in the open air. This reaction doesn’t change the ink’s color, but makes it water-resistant and sets it more permanently on paper. As it turns out, we’re recreating an ink preparation procedure as it was practiced in the Middle Ages and up to the 20th century!