Sugar snake
A black snake grows out of a pile of sugar and baking soda


Reagents
Safety
-
Put protective eyewear on.
-
Conduct the experiment on the tray.
-
Keep a bowl of water nearby during the experiment.
-
Place the stove on the cork hot pot stand. Do not touch the stove after the experiment; wait until it gets cold.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Urotropine (solid fuel) lumped. How to measure it now?
Indeed, this reagent tends to lump when stored. We have foreseen this case and supplied a black ball-tipped rod in the set, which you may use to crush the lumped salt.
The solid fuel is too powdery and doesn’t clump up. What should I do?
Pour the solid fuel into a plastic cup and add 4 drops of water. Mix the wetted powder well and put it back into the mold.
Alternatively, you can also add 3 drops of liquid soap solution from the "Tin" set, which you received with "Chemistry of monsters" kit and perform the same operation.
Then proceed from step 4.
Can you eat or touch the snake?
You must never taste any of the chemicals or products of the reactions that you work with, even if, in theory, it should be safe to do so. Perhaps you got an unexpected product from your reaction, or perhaps the starting chemicals contained impurities. In any case tasting your experiment could be very dangerous.
It is for these reasons that eating in professional laboratories is strictly forbidden. Safety first!
After the "snake" has cooled you can touch it, but be careful, it may be hot. The carbon contained in the "snake" may smolder. The carbon form the "snake" will get onto your hands, so wash your hands after the experiment.
Step-by-step instructions
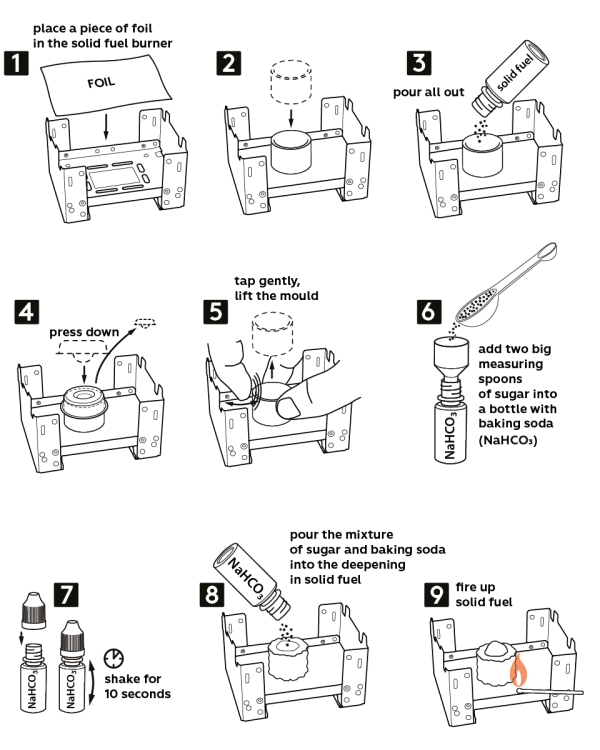
-
Take the solid fuel stove from the Starter kit. Place a piece of aluminum foil onto the stove. Caution! Use a cork hot pad to protect the tabletop.
-
Place the plastic ring in the center of the foil.
-
Pour out all the solid fuel inside the ring (2.5 g).
-
Take the press mold and push it down into the ring to make a crater with the solid fuel. Now, carefully remove the press mold.
-
Remove the plastic ring while slightly tapping onto it.
-
Pour in two level measuring spoons of sugar (2 g) into a vial with 0.5 g of sodium bicarbonate (NaHCO3) and close it with a cap.
-
Shake the vial for 10 seconds to mix sugar and sodium bicarbonate.
-
Now, pour out the mixture of sodium bicarbonate and sugar into the crater you made in solid fuel.
-
Ignite the solid fuel – and very soon, it will grow into a black “snake”!
Expected result
The solid fuel starts to burn. The mixture of sugar and baking soda in the flames will turn into a black "snake." If this experiment is done correctly, it is possible to grow a snake that is 15–50 cm long.
Disposal
Dispose of solid waste together with household garbage.
Scientific description
How does the snake form?
When sugar (C12H22O11) burns (combusts), it turns into water vapor and carbon dioxide. However, complete combustion requires a good oxygen supply. Other complex processes take place at high temperatures, because the flow of oxygen to the inner parts of the pile of sugar is hindered. These processes include the decomposition of sugar to give carbon and water vapor. It is this decomposition to give carbon that gives us a carbon "snake".
Why do we add baking soda (NaHCO3) to the sugar?
Baking soda decomposes when heated to release carbon dioxide (CO2):
2NaHCO3 → Na2CO3 + H2O + CO2
Baking soda is added to bread dough to make it rise, and it is the same for this experiment. The carbon dioxide and water vapor that is given off make the snake light and airy, which allows the snake to grow.
What is this "snake" made of?
The "snake" consists of mainly carbon that comes from the heated sugar, but which was not burnt in the flame. The carbon is what makes the "snake" black. There is also Na2CO3 in the snake, which results from the decomposition of the baking soda when heated.
What chemical reactions occur in the process of formation of the "snake"?
The three main reactions in this process are:
- Combustion of sugar (good oxygen supply available) to give carbon dioxide and water vapor:
С12H22O11 + 12O2 → 12CO2 + 11H2O
- Thermal decomposition of sugar to give carbon and water vapor:
С12H22O11 → 12C + 11H2O
- Thermal decomposition of baking soda to give sodium carbonate, carbon dioxide, and water vapor:
2NaHCO3 → Na2CO3 + CO2 + H2O
That’s interesting!
What is sugar and where does it come from?
A sugar molecule consists of carbon (C), oxygen (O), and hydrogen (H) atoms. This is what a typical sugar, sucrose, looks like:

It is a little hard to make out the structure of sucrose from this image. Download the application MEL Chemistry on your smartphone or tablet so that you can twist and turn the sugar molecule and look at it from different sides to better understand its structure. Look for "sucrose" in the app.
The sucrose molecule is composed of two parts connected to each other by an oxygen atom (O). The two parts are glucose and fructose, which you may have heard of. Glucose and fructose are called simple sugars. Normal, household sugar is called a complex sugar, which means that the sugar molecules consist of several (two) simple sugars.
Here is what the simple sugars look like:
Glucose:

Fructose:

Sugars are important building blocks in plants. Plants produce simple sugars from water and carbon dioxide during photosynthesis. Simple sugars can be linked together to form small molecules, such as sucrose, or to form long chains. Starch and cellulose are examples of long-chain sugars that are made up of simple sugars, also known as polysaccharides. Plants use sugars as building materials and for nutrient storage.
The longer the sugar molecule, the more difficult it is for our bodies to digest it. Therefore we tend to love sweet things, which contain short, simple sugars. But your body was not designed to eat many simple sugars, and in nature they are rare. So be careful of the number of sweets you eat!