Zinc-carbon battery
Make a real zinc-carbon battery!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
All of these items should fit snugly in the tube. Don't be afraid to apply more force if need be. You can also try using another graphite electrode or cotton cylinder. And, of course, you can always ask an adult for help.
You can draw anything you want – let your imagination run wild!
Make sure the screw is touching the metal clamp in the clock mechanism, and the graphite electrode on the opposite end of the battery is touching the metal part of the clock mechanism housing.
If you have assembled the two batteries and connected them correctly to the LED, but the LED isn’t glowing — don’t worry! This is probably easy to fix.
First of all, try switching the wires. Electric current can only run through an LED in one direction. Make sure the crocodile clips are clamped to the metal, not the insulation.
Next, check all the connections. Are all the components of the circuit connected securely? The screw must be touching the springs in the connector, and the graphite rods on the opposite ends of the batteries must be touching the metal parts of the connector housing. Finally, make sure that all the wires are fixed securely to the LED and to the connector.
If none of these steps help, try using a different LED or assembling a new battery.
We do not recommend connecting regular batteries to the LEDs from the kit. This could potentially result in the LEDs overheating and/or malfunctioning.
Step-by-step instructions
Mix some manganese dioxide MnO2 with graphite C. MnO2 is going to "pull" electrons from zinc Zn, while graphite will allow the electrons to travel through the mixture.

Insert a graphite electrode into a silicone tube. This will be the battery body.
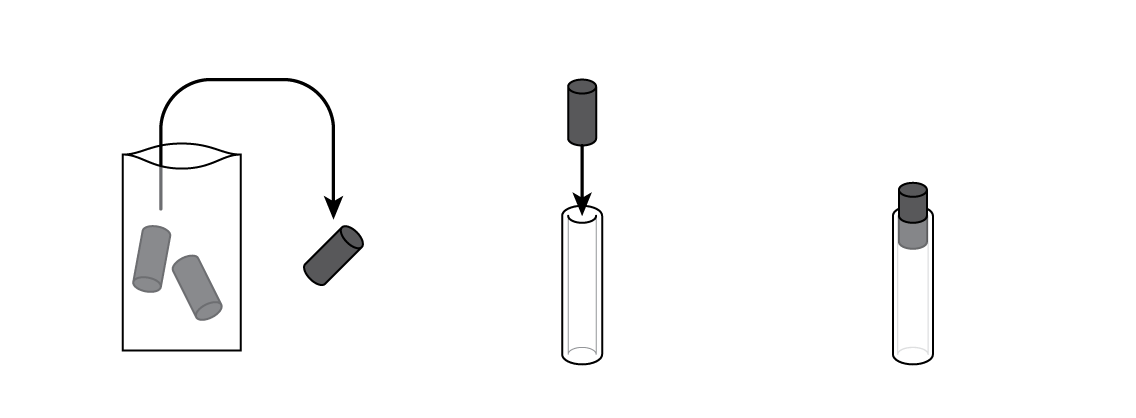
Pour some of your MnO2 and C mixture into your battery cartridge.

Put a cotton cylinder into the cartridge and soak it with ammonium chloride NH4Cl solution. Then insert a bolt plated with zinc Zn—and your battery is ready!
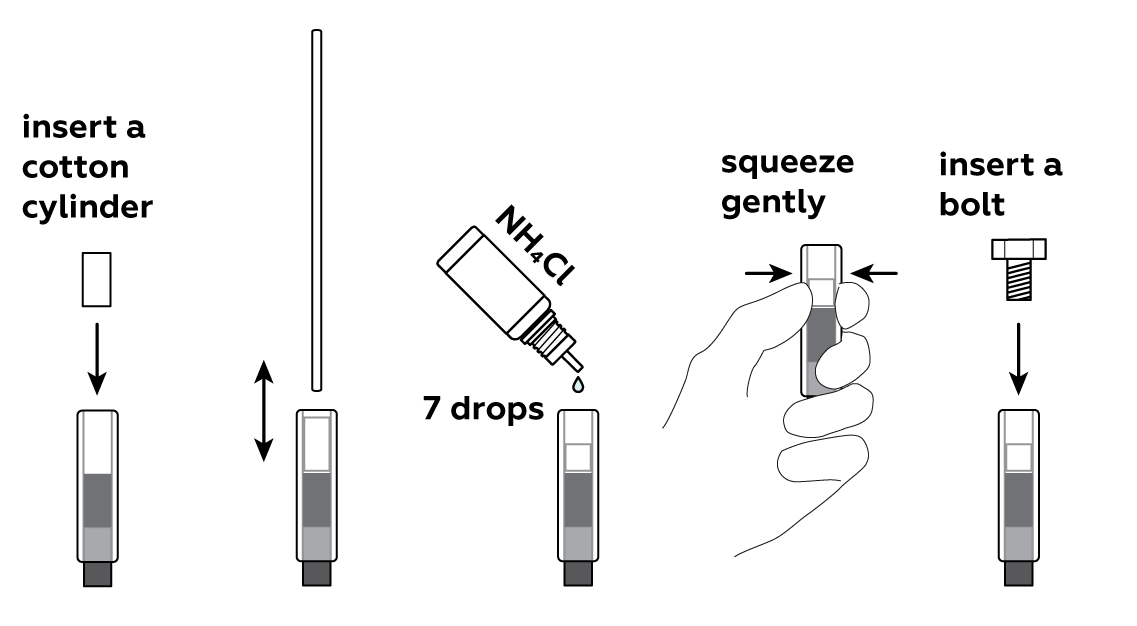
To make use of your newly-made battery, assemble the clock.
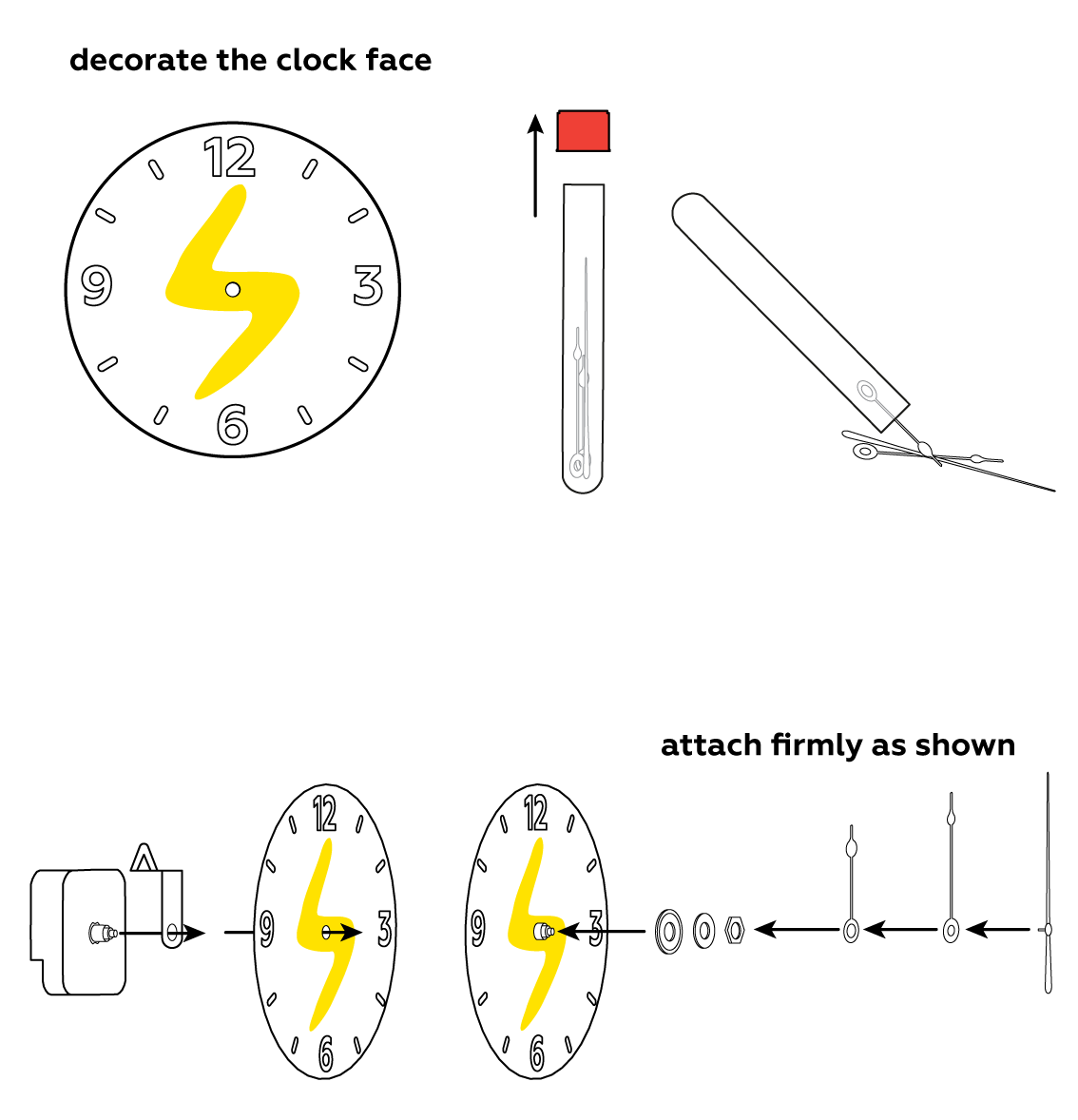
Zn is keen to give its electrons e- away. Electrons are negatively charged, and that's why the Zn side of the battery is connected to the "-" of the clock. MnO2 is willing to take the electrons that zinc gives away, and its side is marked "+". The electrons can't pass through the cotton or the NH4Cl solution, however, so they need another route. Unlike the NH4Cl solution, the clock's internal workings do allow electrons through. But their passage isn't free: in return, the electrons make the clock tick. That's how all electrical devices work.

Assemble another battery and use both of them together to power an LED.

Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description
If the electrons go from zinc Zn to manganese dioxide MnO2, can't you just put the "+" wire of your device
in some MnO2 powder
, connect the "−" wire to a piece of zinc
, and call it a day? Well, not so fast.
As soon as a negatively-charged electron leaves zinc Zn , the latter becomes positively charged and wants to pull the electron back. That is, unless it can get rid of its charge. And that's where the solution of ammonium chloride NH4Cl
in water H2O
comes in.
Metallic Zn can release the positively-charged Zn2+
particles into the solution, and thus get rid of its charge. Something similar happens with MnO2
, which gains negatively-charged electrons e−
. To become uncharged, it ultimately gives its oxygens O
to water H2O
, forming OH−
. Zn2+
and OH−
particles are charged, but they, unlike electrons, can move freely in the solution, so they spread out and mix in the NH4Cl soaked cotton, making it evenly uncharged too.
There are countless ways to make chemicals work to move electrons in wires and this one isn't the simplest. But your battery is chemically identical to commercially produced zinc-carbon batteries. The reason for that is the fortunate combination of readily available ingredients and the lack of free-flowing liquids in the construction of the battery and the absence of gaseous products of the reaction.
How do zinc-carbon batteries work?
Such a battery (also called as zinc-manganese or manganese battery) is a chemical source of electric current that relies on an oxidation-reduction (redox) reaction between manganese dioxide (MnO2) and zinc powder (Zn). Such a reaction involves the transfer of electrons from one element (the reducer) to another element (the oxidizer).
Our battery is divided into two sections, separated by wadding: one section holds the oxidizer MnO2, and the other contains the reductant Zn. When the battery isn’t connected to anything, these isolated substances cannot react with each other. But when the crocodile clips are connected to a diode, the circuit is closed and the reaction can begin: electrons start migrating from the zinc section to the manganese section. They move from the bolt through the springs and the black wire to the diode (which starts glowing!), then continue on through the red wire, and finally through the graphite rod to the manganese dioxide (MnO2) section.
Why do we need the graphite powder?
The battery will work only if the electric current can flow “unobstructed,” so the agents inside the battery must be able to conduct electricity very well.
Unlike graphite, manganese dioxide MnO2 is not a good conductor, but a mixture of MnO2 and graphite powder is a fine conductor for such a battery.
Why do we need the NH4Cl solution?
As electrons move from the zinc section to the manganese dioxide section, they create an electron excess in the latter. Meanwhile, an electron shortage arises in the zinc section as electrons move away from it. These must be balanced for the battery to work reliably for an extended period of time.
Ammonium chloride NH4Cl is primarily intended to be a source of hydrogen ions H+ that can balance out the electron excess in the manganese dioxide MnO2 section.
At the same time, chloride ions Cl- balance out the electron shortage in the zinc section.
Also, on the zinc side, the reaction creates Zn2+, which readily forms insoluble compounds in these conditions. If these compounds accumulate, the electric current will eventually just stop. Once again, ammonium chloride comes to the rescue: the ammonia NH3 obtained during the reaction forms a compound with Zn2+ that is easily soluble in water.
That’s interesting!
What are ordinary batteries made of?
The principal difference between zinc-carbon and alkaline batteries is the type of electrolyte incorporated in the battery. As we already know, zinc-carbon (or “salt”) batteries contain ammonium chloride NH4Cl. This agent is a salt, which is where the informal name “salt battery” comes from. Meanwhile, alkaline batteries contain an alkali solution – alkali metal hydroxides (LiOH, NaOH, KOH). Of the three, potassium hydroxide KOH is used most frequently. The batteries also differ in structure. Alkaline batteries generally work longer than “salt” batteries.
“Salt” and alkaline batteries have different markings. Battery markings (a few letters or numbers on the housing) usually consist of one or two letters and numbers. The numbers designate battery type in terms of shape and size. AA and AAA batteries are marked 03 and 6, respectively. The numbers are preceded by letters; R for “salt” batteries, and LR for alkaline batteries. For instance, an alkaline AA battery will be marked LR03. Lithium batteries are designated by the letters CR. If you have a battery with the first two letters SR or PR – you’re in luck! This means you’re holding a rare silver or zinc-air battery.
Generally speaking, any device that needs electric power can run on zinc-carbon batteries. However, a small battery won’t be enough to power, say, a fridge or a washing machine. Zinc-carbon batteries can power small gadgets, such as flashlights, motors in toy cars, clocks, and watches.
The zinc-carbon battery that we assemble can power an LED, a watch, or a small calculator. Such gadgets only require a small amount of electricity to function reliably.
Usually, a battery similar to the one we assemble provides sufficient energy to make an LED glow intensively for 2–3 hours. If you make an effort and assemble it with extreme precision, such a battery can work for up to 10–12 hours!
Chemically, our battery is exactly the same! The main difference is in the batteries’ general structure. In a commercial zinc-carbon battery, the oxidant (MnO2) and reductant (Zn) layers are much thinner, but they are wide and the surface of the membrane (in our case, wadding) is much larger. Such a battery can produce a stronger current than ours, which is why it can power a wider variety of devices (powerful flashlights, TV remotes, children’s toys, computer mice). Furthermore, such a battery is airtight and doesn’t let the electrolyte (ammonium chloride solution NH4Cl) inside evaporate. In our case, the evaporation of the electrolyte solution is the main reason why our battery runs down so quickly.