Lemon battery
Use a lemon... to light up a diode!
Reagents
Safety
- Put on protective gloves and eyewear.
- Conduct the experiment on the plastic tray.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
Yes, that’s alright. Magnesium is an active metal, and it is reacting with the citric acid in the lemon juice. This reaction yields magnesium citrate and releases hydrogen gas, which is making the hissing sound.
Don't worry; use the piece of the lemon you have. Just make sure that the magnesium and copper aren’t touching each other.
First, make sure you’ve connected the copper wire to the red crocodile clip wire and the magnesium strip to the black crocodile clip wire.
Second, check that the LED is connected correctly: the black crocodile clip should be connected to the short “leg,” and the red clip to the long one.
Step-by-step instructions
Connect magnesium Mg strips and copper Cu wires to the crocodile clips.
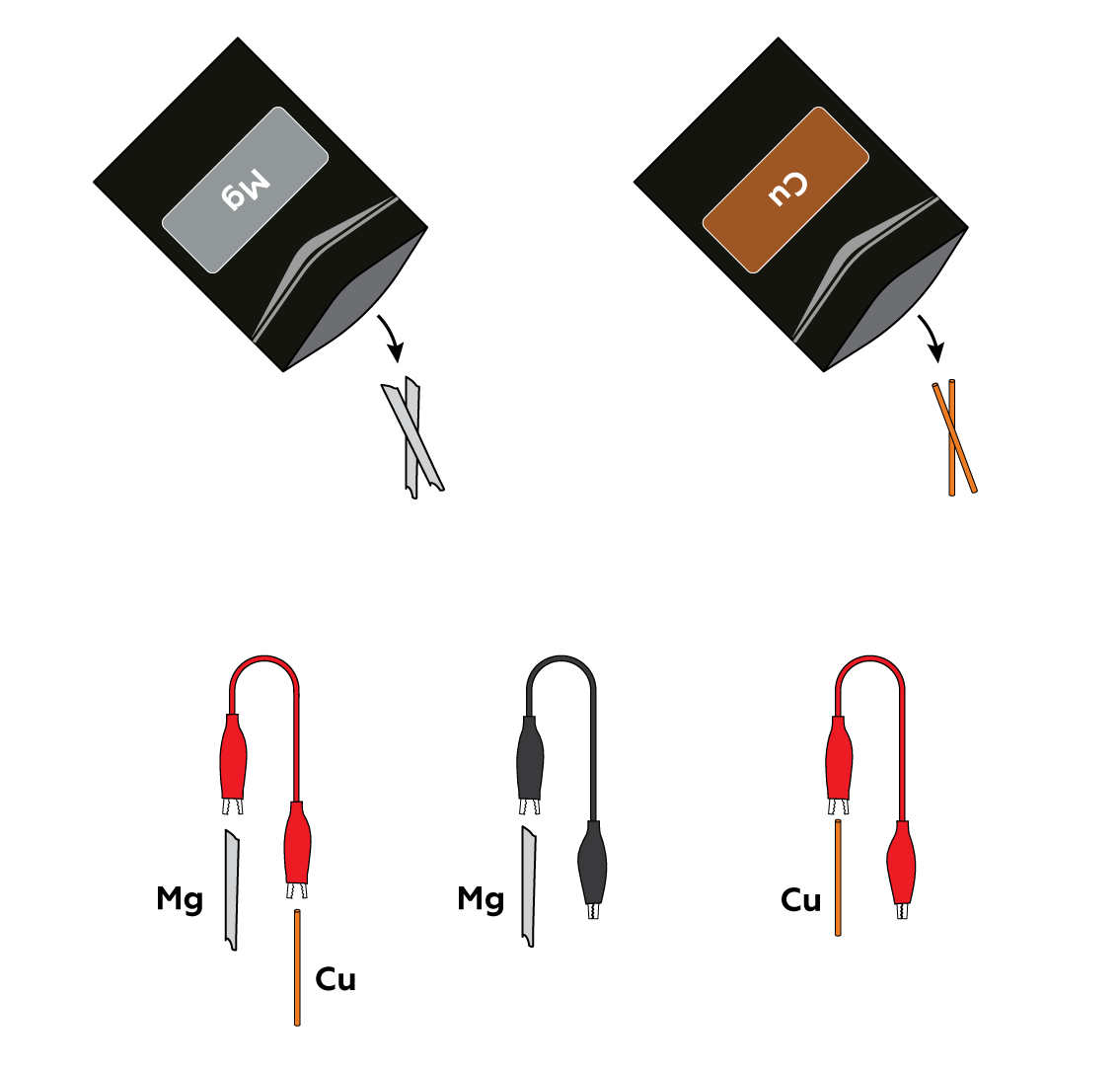
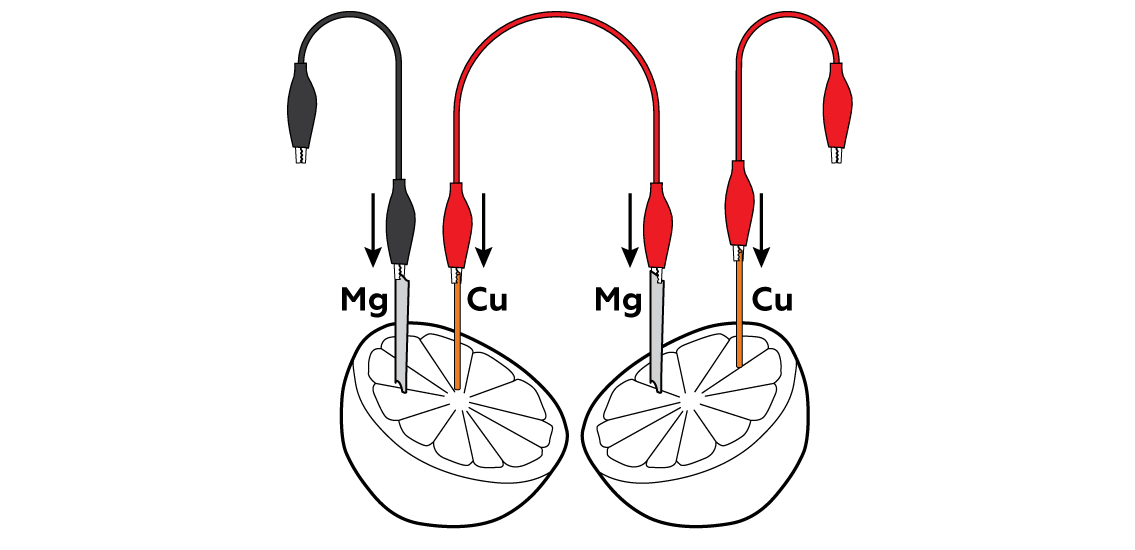
Insert the metals into the pulpy section of the lemon.
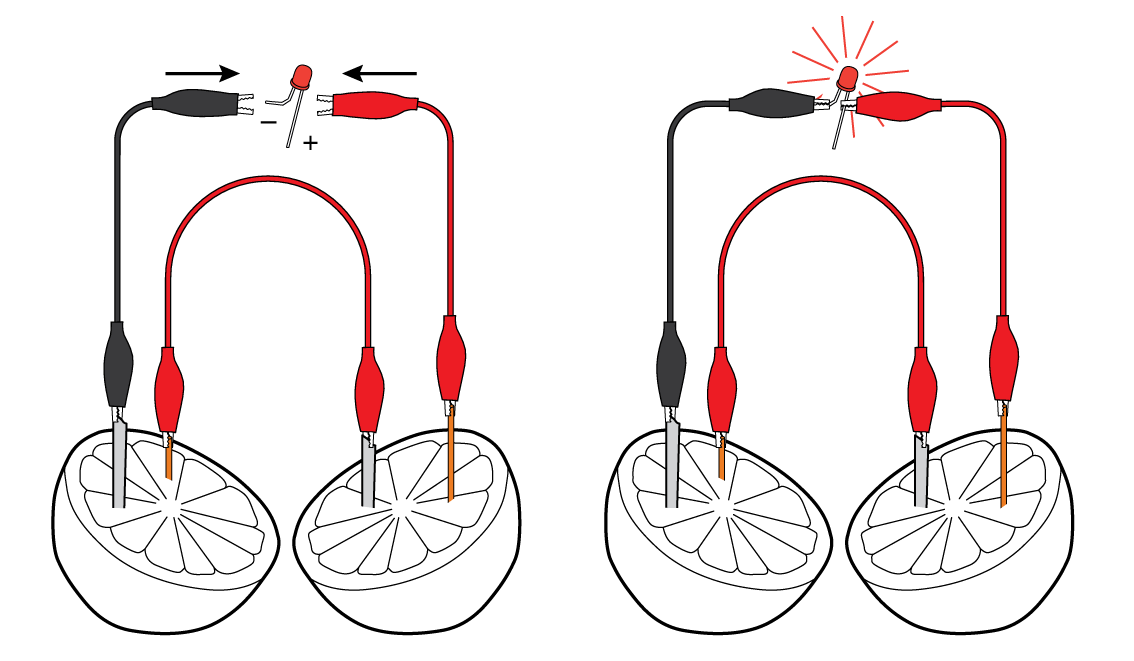
Connect the LED to the crocodile clips. Voila! You’re powering the LED with help of the lemon!
Disposal
Please refer to local regulations when disposing of chemicals. Dispose of other solid waste with household garbage. Pour leftover solutions down the sink. Wash with an excess of water.
Scientific description

What's the secret? Any object contains electrons 






How else can you make your own battery?
What if you desperately needed electricity but didn’t have any copper Cu or magnesium Mg? No worries! Some other pairs of metals would work just fine. To choose a good pair, you can use the chart chemists call the "metal reactivity series." A metal in this series will give electrons to any of the metals to its right (just like magnesium Mg gave electrons to copper Cu), and the farther apart the metals are in the series, the better they will push electrons through the wire.
If, perchance, you're out of lemons too, you can use any juicy fruit, vegetable, or even any solution that contains a lot of ions. Salt water, soda, or juice would work just fine.

How does this cell work?
The working principle of the cell is based on the difference in reactivity between copper and magnesium. Magnesium is a rather reactive metal; its atoms readily get rid of two electrons to form magnesium ions Mg2+. As these magnesium atoms develop a shortage of electrons, the strip develops a positive charge.
Copper is less reactive than magnesium, so if these two metals are included in the same electric cell, the electrons will travel from magnesium to copper – through the LED. This shift is what makes the LED work. Since electrons are negatively-charged particles, the copper wire accumulates an excessive negative charge.
Neither metal is comfortable under such conditions, and here the lemon comes into play. Or, rather, not the lemon itself, but the lemon juice, which contains citric acid. In a solution, citric acid partially dissociates into citrate anions and hydrogen ions H+ (or protons). In other words, it acts as an electrolyte solution – a solution that can conduct electricity. These protons take the excess of electrons from the copper wire to form hydrogen molecules:
2H+ + 2e → H2
At the same time, positively-charged magnesium ions leave the magnesium strip and pass into the solution, which causes the magnesium to gradually dissolve:
Mg0 – 2e → Mg2+
The process will continue until the magnesium strip dissolves completely.
How does an electrolyte solution work?
An electrolyte is usually a substance which can split into ions when dissolved. The resulting solution is called an electrolyte solution. Incidentally, citric acid is not the only substance that can act as an electrolyte. The same can be said for sodium chloride or almost any other water-soluble salt. As both positive ions (called cations) and negative ions (called anions) are released when an electrolyte dissolves, they can help maintain the balance between the charges in the cell, compensating for an excess of negative or positive charge from the metal plates. Without this balance, the battery cannot function.
