TOP 10 chemical reactions that you can repeat at home
Safe and spectacular experiments
1. The power of bubbles
Materials:
-
plastic bottle;
-
150 ml of hot water;
-
yeast;
-
sugar;
-
balloon;
-
teaspoon.
Experiment procedure
- Pour three teaspoons of dry yeast and two teaspoons of sugar into a bottle.
- Slowly pour hot water into the bottle.
- Put the balloon over the bottle and wait for half an hour.
Experiment results
The liquid starts to foam, and the balloon inflates.
Scientific explanation
Yeast is a microscopic fungus that consumes sugar and releases carbon dioxide. The numerous bubbles of this gas rise to the surface, causing the liquid to foam and the balloon to inflate. In chemistry, this process is called fermentation. This particular chemical reaction involves the release of ethyl alcohol and carbon dioxide:
С₆Н₁₂О₆ → 2С₂Н₅ОН + 2СО₂↑

2. Smoke
Materials:
-
ammonia;
-
hydrochloric acid;
-
two strings;
-
two sticks.
Experiment procedure
- Tie the sticks to the strings.
- Lower one string into the bottle of hydrochloric acid, and the other into the ammonia. Let them soak.
- Bring the strings close together.
Experiment results
White smoke begins to appear between them.
Scientific explanation
This experiment relies on the formation of ammonium chloride – the white fumes you see. The hydrochloric acid releases gaseous hydrogen chloride (HCl). The hydrogen chloride reacts with the ammonia (NH₃), and ammonium chloride forms as white “smoke”:
HCl + NH₃ = NH₄Cl
3. Soot (burning)
Materials and tools:
-
candle;
-
lighter;
-
knife.
Experiment procedure
- Light the candle.
- Hold the knife blade in the center of the flame for several seconds.
Experiment results
The blade turns black.
Scientific explanation
Tiny carbon particles, formed as a result of the incomplete combustion of paraffin from the candle, gradually cover the blade:
2С₁₈Н₃₈ (paraffin) + 55О₂ → 36СО₂ + 38Н₂О
4. Release of gas
Materials and equipment:
-
sodium bicarbonate (baking soda);
-
vinegar;
-
water;
-
glass;
-
match.
Experiment procedure
- Fill a glass 1/3 full of water.
- Add a teaspoon of baking soda and a little vinegar.
- Light a match and gently lower it into the glass, not touching the mixture.
Experiment results
The match goes out.
Scientific explanation
Sodium bicarbonate (soda) is a compound of the following elements: sodium, hydrogen, carbon, and oxygen.
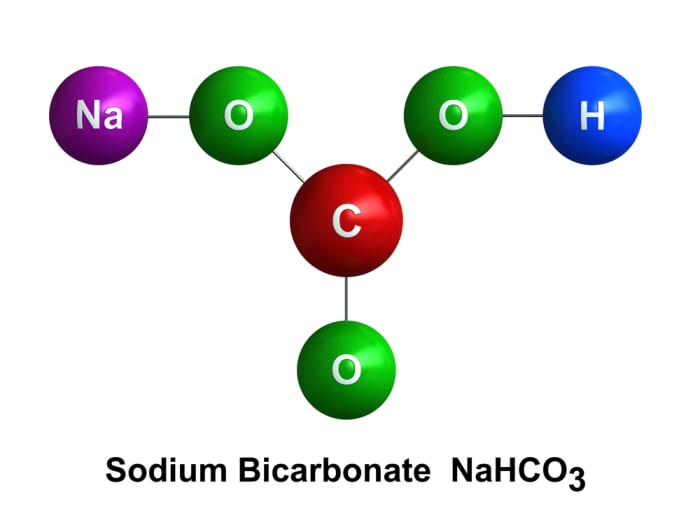
The reaction between sodium bicarbonate and vinegar forms unstable carbonic acid, which immediately decomposes to water and carbon dioxide. The carbon dioxide gas extinguishes the flame:
NaHCO₃ + CH₃COOH → CH₃COONa + H₂O + CO₂↑
5. Destructive vinegar
Materials and equipment:
-
eggshell;
-
vinegar;
-
glass.
Experiment procedure
- Put an eggshell in a glass.
- Fill the glass half full of vinegar. Examine the contents after 12 hours.
Experiment results
The eggshell dissolves in the vinegar.
Scientific explanation
Vinegar is an acidic substance. It has the ability to break down several substances, such as the calcium carbonate contained in the eggshell:
CaCO₃ + 2CH₃COOH → Ca(CH₃COO)₂ + CO₂↑ + H₂O
6. Color experiment with liquid ammonia
Materials:
-
ammonia solution;
-
copper coin.
Experiment procedure
Take a coin with a dark coating and pour liquid ammonia over it.
Experiment results
The solution will turn blue, either immediately or after several minutes.
Scientific explanation
Under the influence of oxygen, copper forms a complex compound with ammonia.
2Cu + 8NH₃ + 2Н₂О + О₂ → 2[Cu(NH₃)₄] (OH)₂
7. Chemical fire
Materials:
-
potassium permanganate crystals;
-
glycerin;
-
water.
Experiment procedure
- Make a small mound of potassium permanganate crystals.
- Make a hollow in them and pour a little glycerin into the hollow.
- If there is no fire, add one or two drops of water.
Experiment results
The mixture catches fire.
Scientific explanation
Potassium permanganate and glycerin enter a reaction accompanied by burning (a flash).
14КМnО₄ + 3С₃Н₅(ОН)₃ → 7K₂CO₃ + 14MnO₂ + 12H₂O↑ + 2CO₂↑
8. Volcano
Materials and tools:
-
flask;
-
water;
-
baking soda;
-
citric acid;
-
dishwashing liquid;
-
container to mix ingredients and spoon.
Experiment procedure
- Fill the flask 2/3 full of water. Add a few drops of dishwashing liquid and five tablespoons of baking soda.
- Dilute citric acid (recommended 5 tablespoons per 1.5 glass of water) in a separate container.
- Stir the mixture in the flask thoroughly. Slowly pour the glass of citric acid into the flask.
Experiment results
Foam starts to pour out of the flask.
Scientific explanation
We get the effect of the foam erupting in the process of a neutralization reaction. When interacting with an alkali (soda), the acid neutralizes it, releasing carbon dioxide, which makes the mixture foam and forces the mass to flow out of the container. The dishwashing liquid makes the “lava” bubble more strongly:
Н₃С₆Н₅О₇ + 3NaHCO₃ → Na₃C₆H₅O₇ + 3CO₂↑ + 3H₂O
Check here to find out how to make a volcano that will glow in the dark.
9. Dissolving polystyrene in acetone
Materials:
-
bowl;
-
small pieces of polystyrene;
-
acetone;
-
rubber gloves.
Experiment procedure
- Put on the gloves.
- Fill the bowl half full of acetone.
- Lower the small pieces of polystyrene into the bowl.
Experiment results
The pieces of polystyrene disappear. Just like this:
Scientific explanation
Polystyrene is expanded styrofoam and primarily consists of air. This is why it dissolves so remarkably in acetone.
10. Invisible ink
Materials:
-
lemon;
-
glass;
-
piece of paper;
-
candle;
-
water;
-
cotton swab.
Experiment procedure
- Squeeze some lemon juice into a glass, add a few drops of water, and mix well.
- Dip a cotton swab into the solution and write on the paper with it. Let the paper dry.
- Hold over a burning candle.
Experiment results
The text appears.
Scientific explanation
Lemon juice contains acid, which darkens at high temperatures.
More experiments you can find in our chemistry sets for kids.