Physical and chemical properties of sodium bisulfate
Reactions with sodium bisulfate, how it’s obtained and applied

Sodium bisulfate NaHSO₄ is the product of the incomplete neutralization of sulfuric acid by sodium hydroxide. It has the formula NaHSO₄. There are several common names of the salt–for example sodium bisulfate or sodium hydrogen sulfate.
Physical properties of sodium bisulfate
Sodium bisulfate is a crystalline substance which forms the crystalline hydrate NaHSO₄·H₂O (a crystalline hydrate is a substance which contains water in the structure of its crystals). In normal conditions, NaHSO₄ constitutes colorless hydroscopic (capable of absorbing water) crystals. The melting point of the crystalline hydrate is 58.5 °C (137.3 °F).
Obtaining sodium bisulfate
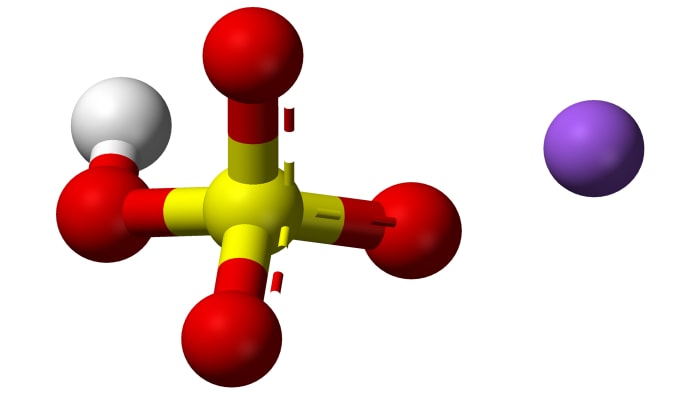
There are several methods for obtaining the acid salt NaHSO₄
1. By the neutralization reaction–the reaction of an acid and a base with the formation of salt and water. Sodium bisulfate may be obtained in the reaction of sulfuric acid and sodium hydroxide, but the substances must be taken in a certain ratio: the acid should be in abundance in relation to the base. The formation of acid salts is an example of the incomplete neutralization of acid:
H₂SO₄ + NaOH = NaHSO₄ + H₂O
(the reaction is carried out with concentrated sulfuric acid, the reagents react in the ratio of 1:1 with the formation of the acid salt sodium bisulfate).
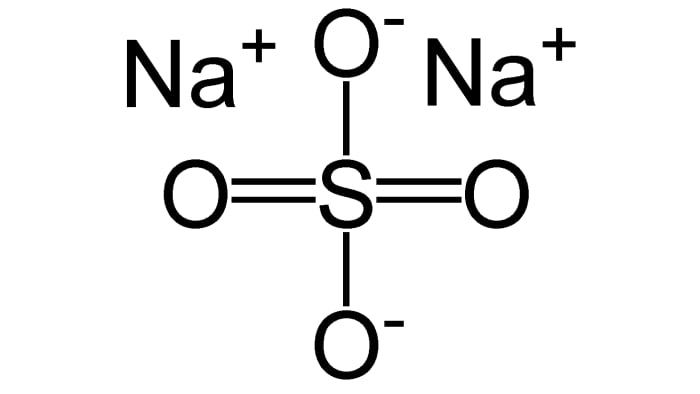
In the reaction of 1 mole of H₂SO₄ and 2 moles of NaOH sodium sulfate Na₂SO₄ forms:
H₂SO₄ + 2NaOH = Na₂SO₄ + 2H₂O
(the protons of sulfuric acid are completely substituted to the sodium ions– complete neutralization takes place, the product is a normal salt)
2. By the reaction between an acid oxide and an alkali (if the oxide is taken in abundance, an acid salt may be obtained):
NaOH + SO₃ = NaHSO₄
If NaOH is taken in abundance in carrying out this reaction, the result will be the normal salt Na₂SO₄.
3. In the reaction of an acid and a normal salt:
Na₂SO₄ + H₂SO₄ = 2NaHSO₄.
Some acid salts (for example NaHCO₃, formed by the weak acid H₂CO₃ and the strong base NaOH) are obtained by hydrolysis (the reaction of a substance with water, in which the initial substance breaks down with the formation of new compounds). Sodium bisulfate cannot be obtained from sulfate by hydrolysis, as this salt is formed by the strong base NaOH and the strong acid H₂SO₄–Na₂SO₄ does not react with water, and only dissolves in it.
Click here for amazing experiments with detailed instructions and scientifical descriptions.
Chemical properties of sodium bisulfate
Many reactions with acid salts allow normal salts to be obtained from them. The chemical properties of sodium bisulfate are the following.
Reaction with hydroxides
NaHSO₄ + NaOH = Na₂SO₄ + H₂O
(adding an alkali to an acid salt makes it possible to obtain the corresponding normal salt, in this case sodium sulfate Na₂SO₄)
If a different alkali is taken, the formation of two salts is possible:
2NaHSO₄ + 2KOH = Na₂SO₄ + K₂SO₄ + 2H₂O
(with mild heating to 42 °C or 107.6 °F)
The reaction may proceed further with the formation of the double salt sodium-potassium sulfate):
Na₂SO₄ + K₂SO₄ = 2KNaSO₄
(the reagents must be present in a ratio of 2:1 by mass respectively)
Reaction with salts
NaHSO₄ + NaCl = Na₂SO₄ + HCl
(reaction takes place with sintering – heating up to 450-800 °C (841-1472 °F) is required, hydrogen chloride HCl is released in gaseous form)
Reaction with oxides
2NaHSO₄ + CuO = CuSO₄ + Na₂SO₄ + H₂O
(reaction takes place with heating up to 450 °C(842 °F)
Heating
2NaHSO₄ = Na₂S₂O₇ + H₂O
(with heating up to 250 °C (482 °F) sodium bisulfate changes to pyrosulfate Na₂S₂O₇)
Sodium bisulfate has found application in industry–for example, it is used in the manufacture of non-ferrous metals, to change poorly soluble compounds into soluble sulfates, and to increase the acidity of water in swimming pools. As a food additive, sodium bisulfate has the code E514.